R
heumatological diseases commonly seen in children
include juvenile idiopathic arthritis (JIA), systemic JIA (sJIA),
Kawasaki disease (KD), Henoch-Schonlein purpura (HSP), systemic lupus
erythematosus (SLE), chronic uveitis, Takayasu arteritis (TA) and
juvenile dermatomyositis (JDM). The initial presentation may overlap
with each other, and even with non-rheumatological disorders such as
infections. The diagnosis of these conditions is primarily clinical.
Laboratory tests can facilitate screening, confirmation of diagnosis,
and monitoring the disease activity and response to treatment. However,
these tests need to be used judiciously and always in context of the
clinical setting [1]. In this review, we describe and discuss various
laboratory tests that are performed in clinical practice.
Basic Laboratory Tests
Hemogram: Anemia is seen in most rheumatological
disorders and is usually indicative of chronicity or disease activity.
Anemia is generally normocytic and normochromic but can be microcytic. A
normal hemoglobin level on follow-up in JIA is reassuring as it
indicates reasonable disease control. Autoimmune disorders like SLE
results in associated autoimmune hemolytic anemia and a positive direct
Coombs test [2]. Total leucocyte count (TLC) is usually increased in
inflammatory disorders like JIA. SLE causes leucopenia specifically,
lymphopenia. A high platelet count is usually indicative of ongoing
inflammation seen in KD, JIA and TA. Fresh onset thrombocytopenia may
indicate macrophage activation syndrome (MAS). Thrombocytopenia in
active lupus disease is commonly associated with antiphospholipid
syndrome (APS) [3].
C-reactive protein (CRP) and Erythrocyte
sedimentation rate (ESR): The CRP and ESR are acute phase
reactants and provide useful information regarding disease activity and
prognostication. Increased ESR may be seen in severe anemia. In
inflammatory disorders it is a reflection of elevated fibrinogen which
is an acute phase reactant. An ESR of more than 100 mm/hour is usually
indicative of a serious underlying disorder like rheumatologic disease,
malignancy or infection. Children with JDM may show a discordance
between disease activity and ESR levels [4]. A rapid fall in ESR with
underlying rheumatological disorder may have sinister connotation as it
may herald the onset of MAS [3]. CRP is a sensitive marker for
inflammation which is synthesized in the liver. It has low specificity
and may be elevated in infections. It reflects changes in disease
activity earlier than ESR. CRP is elevated in active lupus when
associated with ongoing infection, serositis or arthritis [5]. CRP is
elevated in MAS where ESR is usually normal or decreased (Fig.
1) [5,6].
 |
|
Fig. 1 Role of erythrocyte
sedimentation rate (ESR) and C-reactive protein (CRP) in
rheumatological disorders.
|
Urinalysis: Urinalysis is important for
assessment of patients with several rheumatological disorders. Renal
involvement can be a primary manifestation of HSP, SLE, antineutrophilic
cytoplasmic antibodies (ANCA) associated vasculitis and secondary
amyloidosis due to sJIA. KD is the commonest cause of sterile pyuria in
children which can lead to an erroneous diagnosis of a urinary tract
infection [7]. Microscopic hematuria, proteinuria and casts are
suggestive of active disease in SLE nephritis. A 24-hour urinary protein
quantification indicates severity of lupus nephritis. Frequent urine
examination is recommended for early identification of amyloidosis in
patients with sJIA and polyarticular JIA.In HSP, significant renal
involvement is usually seen only in children above the age of 7 years.
In some children with HSP, the renal involvement may not manifest at
first presentation, and may become apparent only on follow-up [8]. Urine
examination in HSP is recommended weekly for first 1 month and every 2-4
weeks thereafter till 6 months [9].
Serum ferritin: Ferritin is an acute phase
reactant and correlates with disease activity. It can differentiate sJIA
and other inflammatory disorders like KD where serum ferritin levels are
significantly elevated in sJIA with normal or slightly raised levels in
KD [10]. Serum ferritin levels are helpful in early identification of
MAS [11].
Rheumatoid factor (RF): RF is an IgM
antibody (but may be IgG, IgA or IgE as well) directed against the Fc
portion of IgG. It is a nonspecific inflammatory marker that can be
elevated in infections like tuberculosis, infective endocarditis,
hepatitis C infection, osteomyelitis and in connective tissue disorders.
The usual methods for estimation of RF are nephelometry and
enzyme-linked immunosorbent assays (ELISA). It is useful in
classification of JIA and prognostication of polyarticular JIA. A
positive RF may suggest onset of erosive disease. According to
International League of Associations for Rheumatology (ILAR), RF should
be positive on two separate occasions for the diagnosis of RF positive
JIA (polyarthritis) as transient elevation of RF is seen in infectious
illnesses. A positive RF does not indicate rheumatoid arthritis .
Anti-Citrullinated peptide antibodies (ACPA):
These antibodies were originally identified in rheumatoid arthritis and
are a marker of severe disease course . While the role of these
antibodies in diagnosis and prognostication of adult rheumatoid
arthritis is well established their role in JIA is still not clear.
Approximately 20% children with JIA can have raised ACPA titres. ACPA
titres are high in RF positive polyarticular JIA. ACPA positivity
indicates more aggressive form of JIA and earlier onset of RF negative
JIA [12,13].
Synovial fluid analysis: Synovial fluid is
present in small quantity in normal joints. Synovial fluid examination
is primarily used for ruling out infections or crystal arthropathy. The
latter is extremely rare in children [14]. The aspirated fluid should be
subjected to total and differential leucocyte counts, protein and
glucose estimation, Gram stain and culture. Leucocyte count is helpful
in differentiating between non-inflammatory arthritis (<2000/mm
3)
and inflammatory arthritis (³2000/mm3).
Leucocyte count in synovial fluid in children with active JIA may be as
high as 50,000-100,000/mm3
with neutophilic predominance, decreased glucose, increase protein and
low complements which can mimic septic arthritis [14]. Several studies
have shown that there are differences in cytokine and proteomic profiles
in synovial fluids of different JIA subtypes [15,16]. Synovial fluid
CD4+:CD8+ T cell ratio reversal and increased levels of CCL5 chemokine
predicts development of extended oligoarthritis. Increased IL-18 levels
in synovial fluid predicts activity in systemic arthritis. Rarely
synovial fluid can be hemorrhagic in hemophilia, pigmented villonodular
synovitis or hemagioma. Synovial biopsy is helpful in diagnosis of
tuberculous arthritis.
Muscle enzymes: Muscle enzymes can be elevated in
inflammatory myopathies like JDM and include creatine kinase (CK),
lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and
aldolase. All muscle enzymes may not be elevated in a patient at the
same time. Serum levels of these enzymes usually decrease 3-4 weeks
prior to clinical improvement in muscle strength. Similarly, an increase
in muscle enzymes can predict disease relapse 5-6 weeks prior to
clinical manifestations. CK is the first to rise and first to fall. A
combination of LDH and AST is the best predictor for disease activity in
children with JDM [17].
Complement system: The complement system
represents a cascade of more than 30 proteins and has 3 distinct
pathways for activation viz (a) classical pathway, (b)
mannose-binding lectin (MBL) pathway and (c) alternative pathway.
All three pathways result in membrane attack complex formation and cell
lysis. There are two broad methods for screening of complement system: (a)
measurement of individual complement level (b) functional assay
to assess functional ability of whole complement pathway (CH
50)
or alternative pathway (AH50).
CH50 testing effectively
screens for diseases of whole complement system except MBL and factor B
or D of alternative pathway. In classical pathway activation both C3 and
C4 will be low while low C3 and a normal C4 indicate alternative pathway
activation [15]. Low C3, C4 is an important clue to diagnosis of SLE.
Assay of complements also serves to evaluate disease activity and
treatment response in patients with lupus as decreased C3 or C4 levels
correlate with disease flare. Complement levels normalize with disease
improvement Complement deficiency (inherited) can also be associated
with early onset lupus [16]. Lupus due to complement deficiency has
early onset of disease (usually <7 years of age) and has a predilection
for the central nervous system [16].
Autoantibodies
The presence of autoantibodies provide important
clues to diagnosis of rheumatological disorders. Tests that are commonly
used in clinical practice include ANA, anti-dsDNA, Immunoblot assay,
Anti-neutrophil cytoplasmic antibody (ANCA) and anti-phospholipid
antibodies (APLs).
Anti-nuclear antibody (ANA): Most commonly used
methods for detection of ANA are indirect immunofluorescence (IIF) and
ELISA based assays. Gold standard for ANA detection is IIF based assay
which is performed by incubation of the test serum with a substrate that
allows antibody binding. Anti-human IgG antisera labelled with a
flurochrome is added (Fig. 2). The preferred substrate is
human epithelial (HEp-2) cells, although in the past this test was
carried out on rat liver cells. The IIF test also shows the pattern and
staining intensity of ANA positivity. The ANA pattern is dependent on
the specific nuclear antigens that provide the substrate (Fig.
3) [18]. ELISA is not the preferred method for detection of ANA and
can be negative in at least a third of patients who test positive by IIF
[18,19]. It allows high throughput of samples. The results are dependent
upon specific nuclear antigens in the test system and can be false
negative when a patient has an ANA that is not present in the commercial
kit being used. A positive ANA is not synonymous with a diagnosis of
lupus. Studies have shown that 9-15% healthy children can have positive
ANA [20]. Results of ANA, therefore, must be interpreted in context of
clinical findings [18]. ANA has very good negative predictive value but
carries low specificity. Patients with other rheumatological disorders (e.g.
scleroderma, mixed connective tissue disorders and overlap syndrome) can
be ANA positive. In patients with oligoarticular JIA, positive ANA
predicts risk of uveitis [21]. ANA positivity can also predict the risk
of development of underlying connective tissue diseases in children
presenting with Raynaud phenomenon [22].
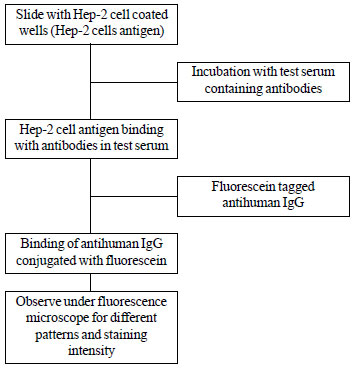 |
|
Fig. 2 Indirect immunofluorescence
method for measuring Anti-nuclear antibody (ANA).
|
 |
|
Fig. 3 Different ANA patterns and
association with disorders.
|
Anti-double-stranded DNA (Anti dsDNA) antibodies:
These antibodies are highly specific and confirmatory for SLE.
Measurement of these antibodies can be performed by various methods
which include IIF and ELISA. Most specific method for estimation of
anti-dsDNA is Crithidia lucilae based IF. It is highly specific for SLE
but has poor negative predictive value. Elevated anti-dsDNA titers
correlate well with active lupus nephritis and can be used to monitor
disease activity [18,23,24].
Immunoblot assay: Immunoblotting is based on the
principle of Western blotting where protein antigens (including nuclear
and cytoplasmic antigens) are separated by using polyacrylamide gel
electrophoresis. These antigens are then transferred to a nitrocellulose
strip that is incubated with test serum. If antibody is present in the
serum, it binds to a specific antigen over the membrane strip and that
can be recognized by comparison with control results. The test will miss
an antigen if not present in the commercial kit unlike IIF [18].
Anti-Neutrophil cytoplasmic antibodies (ANCA):
ANCA are auto-antibodies against different antigenic components of
azurophilic granules in neutrophils. ANCA is performed by IIF and ELISA
based assays [25]. IIF is a more sensitive while ELISA is more specific.
There are different IIF patterns of ANCA- cytoplasmic ANCA (c-ANCA);
perinuclear ANCA (p-ANCA); and atypical ANCA. c-ANCA positivity results
for binding to proteinase 3 (PR3) while p-ANCA positivity results for
binding to Myeloperoxidase (MPO) enzyme. ELISA assay is used to confirm
presence of specific antibody to PR-3 and MPO [18,26]. c-ANCA positivity
is associated with granulomatosis with polyangiitis (GPA) while p-ANCA
is positive in microscopic polyangiitis (MPA) and eosinophilic
granulomatosis with polyangiitis (EGPA).
Anti-phospholipid antibodies (APLs): The term
APLs refers to a group of autoantibodies directed against phospholipid
or plasma binding proteins. The three most important APLs that are
screened are- lupus anticoagulant (LA); anti-
b2
glycoprotein-I antibodies (anti-b2GPI)
IgG and/or IgM; anticardiolipin antibodies (aCL) IgG and/or IgM. APS is
an autoimmune syndrome and commonest prothrombotic disorder in children.
APS is characterized by vascular thrombosis or pregnancy morbidity with
persistently positive APLs. APS may occur as de novo (primary APS) or
secondary to an autoimmune disorder (e.g. lupus). The prognosis
and management of APS is determined by the type, number, and titer of
specific APLs. APL positivity is defined with at least one of the
antibodies positive twice at least 12 weeks apart (Box I).
It is recommended that all children with lupus should be screened for
APLs at baseline and then annually [27,28].
|
BOX I Common Anti-phospholipid Antibodies |
|
Lupus anticoagulant
• Best correlation with
thrombotic events
• Defer testing if a patient
has been started on anticoagulants
Anticardiolipin antibodies (aCL)
IgG and IgM
• High titer positivity of
IgG or IgM aCL on two or more occasions, at least 12 weeks apart
• High sensitivity for the
APS syndrome, but specificity is low
• High titre of IgG aCL is
suggestive of APS, while an elevated isolated IgM aCL is
frequently detected in infectious diseases
• Transient/mild elevation
is not of clinical significance. Titers to be repeated after 12
weeks
Anti- b2
glycoprotein-I antibodies IgG and IgM
• High titer positivity of
IgG or IgM anti- b2GPI
on two or more occasions at least 12 weeks apart
• Highest sensitivity for
predicting APS
• Domain-I antibodies are
strongly associated with thrombus formation
Triple positivity
• Positive predictive value for APS is
highest when all three APLs are positive
|
Imaging
While there are no specific imaging findings that can
suggest diagnosis of JIA, it is helpful in disease assessment and
monitoring [29] (Table I).
TABLE I Imaging in Pediatric Rheumatology
|
Imaging
|
Role in Pediatric Rheumatology |
|
X-rays (in JIA) |
•Help in rule out structural damage |
|
•Help in ruling out other possible causes of joint pain and
swelling, such as trauma, skeletal abnormalities, and bone
tumors |
|
Limitations |
|
•Soft tissue changes are not identified
|
|
•Virtually no role in diagnosis of JIA |
|
Ultrasound imaging of joints
|
•Non-invasive, no radiation exposure, and can be repeated
frequently |
|
•Helpful in detection of tenosynovitis, enthesitis, cartilage
and bone abnormalities |
|
•Can detect inflammation and early bone erosions
|
|
•Facilitates intra-articular injections |
|
Limitations:
|
|
•Highly operator dependent; requires skill and experience
|
|
•Interpretation in growing skeleton needs comprehensive
knowledge of anatomical details at different ages |
|
MRI of musculoskeletal system |
•Ideal imaging modality for assessment of pathology of soft
tissues (muscles, tendons, ligaments, fasciae), bones, and
joints (especially during early stage of arthritis). Muscle
involvement assessed in JDMS. |
|
•Gold standard modality for identification of subclincal
disease, monitoring and to see response to therapeutics in
patients with JIA |
|
•Special value in assessment for certain joints including
temporomandibular joint, axial involvement (cervical spine-
atlantoaxial instability, JIA with cervical spine involvement,
and spinal cord compression) and sacroiliac joint involvement in
HLA-B27 related arthritis
|
|
Limitations |
|
•Is an expensive modality; may not be readily accessible |
|
•Longer scan times mandates sedation in young children |
|
•Expertise in interpretation of musculoskeletal MRI may not be
easily available |
|
2D-echocardiography
|
KD: assessment of coronary artery involvement and other cardiac
manifestations Takaysu arteritis: assessment of cardiac
dysfunction
|
|
CT angiography
|
Non-invasive method to asses degree of vascular involvement in
Takayasu arteritis/ polyarteritis nodosa |
|
Dual source CT coronary angiography
|
Assessment of coronary artery abnormalities in KD
|
|
PET scan |
For assessment of disease activity in large vessels in Takayasu
arteritis. |
|
JIA: Juvenile idiopathic arthritis, JDMS: Juvenile
dermatomyosistis syndrome, KD: Kawasaki Disease. |
Biomarkers
KD: Diagnosis of KD is largely clinical and no
laboratory gold standard is available. Studies have shown that levels of
interleukin (IL)-6, IL-17, IL-20 and tumor necrosis factor-a (TNF-a)
are elevated in acute phase of disease [30]. Cardiac biomarker N
terminal pro-B-type natriuretic peptide (NT-proBNP) is elevated in
children with KD during the acute phase and is of value when the
clinical presentation is incomplete or atypical [30-32]. Procalcitonin
is a good marker to differentiate between viral infections and KD. High
serum procalcitonin values are associated with increased risk of IVIg
resistance in KD [33].
HSP: Renal involvement in HSP is vital for long
term outcome. A rapid decline of factor XIII in HSP with severe
gastrointestinal involvement is associated with greater risk of renal
involvement [34]. Elevated D-dimers, elevated urinary monocyte
chemotactic protein-1/creatinine ratio, and HLA-B35 has been found to
associated with higher chances of development of HSP nephritis [35-37].
JIA: Increased levels of S100 proteins and IL-18
are associated with disease activity and predict therapeutic response in
sJIA. Increased levels of soluble CD163, soluble IL 2 receptor-
1. Spencer CH, Patwardhan A. Pediatric rheumatology
for the primary care clinicians-recognizing patterns of disease. Curr
Probl Pediatr Adolesc Health Care. 2015;45:185-206.
2. Castro C, Gourley M. Diagnostic testing and
interpretation of tests for autoimmunity. J Allergy Clin Immunol.
2010;125:S238-47.
3. Ravelli A, Minoia F, Davì S, Horne A, Bovis F,
Pistorio A, et al. 2016 classification criteria for macrophage
activation syndrome complicating systemic juvenile idiopathic arthritis:
A European League Against Rheumatism/American College of Rheumatology/Paediatric
Rheumatology International Trials Organisation Collaborative Initiative.
Ann Rheum Dis. 2016;75:481-9.
4. Breda L, Nozzi M, De Sanctis S, Chiarelli F.
Laboratory tests in the diagnosis and follow-up of pediatric rheumatic
diseases: an update. Semin Arthritis Rheum. 2010;40:53-72.
5. Dima A, Opris D, Jurcut C, Baicus C. Is there
still a place for erythrocyte sedimentation rate and C-reactive protein
in systemic lupus erythematosus? Lupus. 2016;25:1173-9.
6. Ravelli A, Davì S, Minoia F, Martini A, Cron RQ.
Macrophage Activation Syndrome. Hematol Oncol Clin North Am.
2015;29:927-41.
7. Pilania RK, Bhattarai D, Singh S. Controversies in
diagnosis and management of Kawasaki disease. World J Clin Pediatr.
2018;7:27-35.
8. Singh S, Devidayal, Kumar L, Joshi K, Minz RW,
Datta U. Severe henoch-schönlein nephritis: Resolution with azathioprine
and steroids. Rheumatol Int. 2002;22:133-7.
9. Watson L, Richardson ARW, Holt RCL, Jones CA,
Beresford MW. Henoch schonlein purpura-a 5-year review and proposed
pathway. PloS One. 2012;7:e29512.
10. Mizuta M, Shimizu M, Inoue N, Kasai K, Nakagishi
Y, Takahara T, et al. Serum ferritin levels as a useful
diagnostic marker for the distinction of systemic juvenile idiopathic
arthritis and Kawasaki disease. Mod Rheumatol. 2016;26:929-32.
11. Singh S, Chandrakasan S, Ahluwalia J, Suri D,
Rawat A, Ahmed N, et al. Macrophage activation syndrome in
children with systemic onset juvenile idiopathic arthritis: clinical
experience from northwest India. Rheumatol Int. 2012;32:881-6.
12. Spârchez M, Miu N, Bolba C, Iancu M, Spârchez Z,
Rednic S. Evaluation of anti-cyclic citrullinated peptide antibodies may
be beneficial in RF-negative juvenile idiopathic arthritis patients.
Clin Rheumatol. 2016;35:601-7.
13. Tebo AE, Jaskowski T, Davis KW, Whiting A,
Clifford B, Zeft A, et al. Profiling anti-cyclic citrullinated
peptide antibodies in patients with juvenile idiopathic arthritis.
Pediatr Rheumatol Online J. 2012;10:29.
14. Akikusa J, Choo S. Laboratory investigations. In:
Ross E. Petty, Ronald M. Laxer, Carol B. Lindsley, Lucy R. Wedderburn,
editors. Textbook of Pediatric Rheu-matology. Seventh Ed. 1600 John F.
Kennedy Blvd. Ste 1800 Philadelphia, PA 19103-2899: Elsevier; P.
117-28.
15. Singh S, Bansal A. Twelve years experience of
juvenile dermatomyositis in North India. Rheumatol Int. 2006;26:510-5.
16. Vignesh P, Rawat A, Sharma M, Singh S. Complement
in autoimmune diseases. Clin Chim Acta Int J Clin Chem. 2017;465:123-30.
17. Bhattad S, Rawat A, Gupta A, Suri D, Garg R, de
Boer M, et al. Early complement component deficiency in a
single-centre cohort of pediatric onset lupus. J Clin Immunol.
2015;35:777-85.
18. Saikia B, Rawat A, Vignesh P. Autoantibodies and
their Judicious Use in Pediatric Rheumatology Practice. Indian J Pediatr.
2016;83:53-62.
19. Meroni PL, Schur PH. ANA screening: An old test
with new recommendations. Ann Rheum Dis. 2010;69:1420-2.
20. Hilário MOE, Len CA, Roja SC, Terreri MT, Almeida
G, Andrade LEC. Frequency of antinuclear antibodies in healthy children
and adolescents. Clin Pediatr (Phila). 2004;43:637-42.
21. Solomon DH, Kavanaugh AJ, Schur PH, American
College of Rheumatology Ad Hoc Committee on Immunologic Testing
Guidelines. Evidence-based guidelines for the use of immunologic tests:
antinuclear antibody testing. Arthritis Rheum. 2002;47:434-44.
22. Falcini F, Rigante D, Candelli M, Martini G,
Corona F, Petaccia A, et al. Anti-nuclear antibodies as predictor
of outcome in a multi-center cohort of Italian children and adolescents
with Raynaud’s phenomenon. Clin Rheumatol. 2015;34:167-9.
23. Singh S, Abujam B, Gupta A, Suri D, Rawat A,
Saikia B, et al. Childhood lupus nephritis in a developing
country-24 years’ single-center experience from North India. Lupus.
2015;24:641-7.
24. Abujam B, Gupta A, Suri D, Rawat A, Singh S.
Trends and predictors of mortality in childhood onset lupus in a single
North-Indian centre over 23 years: A retrospective study. Clin Exp
Rheumatol. 2016;34:554-9.
25. Csernok E, Holle JU. Twenty-eight years with
antineutrophil cytoplasmic antibodies (ANCA): how to test for ANCA —
evidence-based immunology? Auto-Immun Highlights. 2010;1:39-43.
26. Bossuyt X, Cohen Tervaert J-W, Arimura Y,
Blockmans D, Flores-Suárez LF, Guillevin L, et al. Position
paper: Revised 2017 international consensus on testing of ANCAs in
granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev
Rheumatol. 2017;13:683-92.
27. Ahluwalia J, Singh S, Naseem S, Suri D, Rawat A,
Gupta A, et al. Antiphospholipid antibodies in children with
systemic lupus erythematosus: A long-term clinical and laboratory
follow-up status study from Northwest India. Rheumatol Int.
2014;34:669-73.
28. Groot N, de Graeff N, Avcin T, Bader-Meunier B,
Dolezalova P, Feldman B, et al. European evidence-based
recommendations for diagnosis and treatment of paediatric
antiphospholipid syndrome: The SHARE initiative. Ann Rheum Dis.
2017;76:1637-41.
29. Colebatch-Bourn AN, Edwards CJ, Collado P,
D’Agostino M-A, Hemke R, Jousse-Joulin S, et al. EULAR-PReS
points to consider for the use of imaging in the diagnosis and
management of juvenile idiopathic arthritis in clinical practice. Ann
Rheum Dis. 2015;74:1946-57.
30. Rawat A, Singh S. Biomarkers for diagnosis of
Kawasaki disease. Indian Pediatr. 2015;52:473-4.
31. Reddy M, Singh S, Rawat A, Sharma A, Suri D,
Rohit MK. Pro-brain natriuretic peptide (ProBNP) levels in North Indian
children with Kawasaki disease. Rheumatol Int. 2016;36:551-9.
32. Dionne A, Meloche-Dumas L, Desjardins L, Turgeon
J, Saint-Cyr C, Autmizguine J, et al. N-terminal pro-B-type
natriuretic peptide diagnostic algorithm versus American Heart
Association algorithm for Kawasaki disease. Pediatr Int Off J Jpn
Pediatr Soc. 2017;59:265-70.
33. Dominguez SR, Martin B, Heizer H, Jone P-N, Tong
S, Davidson J, et al. Procalcitonin (PCT) and Kawasaki disease:
Does PCT correlate with ivig-resistant disease, admission to the
intensive care unit, or development of coronary artery lesions? J
Pediatr Infect Dis Soc. 2016;5:297-302.
34. Kawasaki Y, Ono A, Ohara S, Suzuki Y, Suyama K,
Suzuki J, et al. Henoch-Schönlein purpura nephritis in childhood:
Pathogenesis, prognostic factors and treatment. Fukushima J Med Sci.
2013;59:15-26.
35. Fuentes Y, Hernández AM, García-Roca P, Valverde
S, Velásquez-Jones LF, Sosa G, et al. Urinary MCP-1/creatinine in
Henoch-Schönlein purpura and its relationship with nephritis. Pediatr
Nephrol Berl Ger. 2014;29:1047-52.
36. Mahajan N, Kapoor D, Bisht D, Singh S, Minz RW,
Dhawan V. Levels of interleukin-18 and endothelin-1 in children with
Henoch-Schönlein purpura: A study from northern India. Pediatr Dermatol.
2013;30:695-9.
37. Mahajan V, Singh S, Khullar M, Minz RW. Serum and
urine nitric oxide levels in children with Henoch-Schonlein purpura
during activity and remission: A study from North India. Rheumatol Int.
2009;29:1069-72.
38. Consolaro A, Varnier GC, Martini A, Ravelli A.
Advances in biomarkers for paediatric rheumatic diseases. Nat Rev
Rheumatol. 2015;11:265-75.
39. Swart JF, de Roock S, Prakken BJ. Understanding
inflammation in juvenile idiopathic arthritis: How immune biomarkers
guide clinical strategies in the systemic onset subtype. Eur J Immunol.
2016;46:2068-77.
40. Hussain A, Rawat A, Jindal AK, Gupta A, Singh S.
Autoantibodies in children with juvenile dermatomyositis: A single
centre experience from North-West India. Rheumatol Int. 2017;37:807-12.
41. Alibaz-Öner F, Aydýn SZ, Direskeneli H. Recent
advances in Takayasu’s arteritis. Eur J Rheumatol. 2015;2:24-30.
42. Onouchi Y. The genetics of Kawasaki disease. Int
J Rheum Dis. 2018;21:26-30.
43. Chheda P, Warghade S, Mathias J, Dama T, Matkar
S, Shah N, et al. HLA-B27 testing: A journey from flow cytometry
to molecular subtyping. J Clin Lab Anal. 2018;32:e22382.
44. Weiss PF. Update on enthesitis-related arthritis.
Curr Opin Rheumatol. 2016;28:530-6.
45. Singh S, Bhattad S, Danda D. Genetics of juvenile
idiopathic arthritis. Int J Rheum Dis. 2014;17:233-6.
46. Costa-Reis P, Sullivan KE. Monogenic lupus: It’s
all new! Curr Opin Immunol. 2017;49:87-95.
47. Ozen S, Eroglu FK. Pediatric-onset Behçet
disease. Curr Opin Rheumatol. 2013;25:636-42.
48. Demirkaya E, Consolaro A, Sonmez HE, Giancane G,
Simsek D, Ravelli A. Current research in outcome measures for pediatric
rheumatic and autoinflammatory diseases. Curr Rheumatol Rep. 2016;18:8.
49. Brunner HI, Ravelli A. Developing outcome
measures for paediatric rheumatic diseases. Best Pract Res Clin
Rheumatol. 2009;23:609-24.

