Endotracheal intubation is a commonly performed
procedure in neonates. Optimum placement of endotracheal tube (ET) in
the trachea requires high degree of precision. Mal-placement of ET
results in complications including pneumothorax, lung collapse, tracheal
damage and unplanned extubation [1]. Placement of ET up to optimum depth
(insertional length, IL) has been predicted based on various
anthropometric parameters such as weight, gestation, sternal length
(SL), nasal tragus length (NTL), occipital frontal circumference (OFC),
crown heel length (CHL) and foot length [2-4]. However, despite using
clinical predictors of IL, mal-position of ET has been reported to be as
high as 58% [5]. The gold standard to confirm ET position is chest
radiograph. However, radiograph carries disadvantage of radiation
exposure, excessive handling of sick infants and time delay [6]. In
addition, it may not be feasible to use X-ray when duration of
intubation is brief e.g., during surfactant administration.
Point of care ultrasound (USG) has been found to be a
safe and feasible modality to determine ET tip position in neonates
[7-11]. An ET tip placed
0.5-1 cm above the arch of aorta suggests its correct placement [7,11];
though, it has been defined irrespective of weight and gestation
[16,20]. On the contrary, the length of the trachea has been reported to
be variable with weight, length and gestation [12-14].
Therefore, the present study was planned to derive
normative data of the distance between optimally placed ET tip and arch
of aorta across different birthweights and gestations by USG.
Methods
The study was conducted in the neonatal intensive
care unit (NICU) of a tertiary-care centre in northern India from April
2015 to May 2016. All neonates admitted in NICU were screened for
eligibility. Infants with known tracheal, esophageal, cardiac and cranio-facial
anomalies and those with generalized edema were excluded. Informed
consent was obtained from either parent of the infants, who were
presumed to be at risk of intubation by the treating neonatologist.
Following intubation, infants were re-assessed by the principal or
co-investigator/s based on detailed clinical examination, chest X-ray
or echocardiographic findings. Subjects found to be unsuitable for any
of the anthropometric examination or ultrasound measurement including
even minor abnormalities such as low set ear or depressed nasal bridge
were further excluded. Each eligible infant was enrolled only once
during the study period. The study was approved by the Institutional
ethics committee and registered with Clinical trial registry of India
(CTRI). The primary objective of the study was to measure the distance
between optimally-placed ET tip and arch of aorta by USG across
different weights and gestations. Secondary objective was to find out
correlation between IL of optimally placed ET and various anthropometric
parameters: weight, OFC, CHL, NTL and SL.
All intubations were done through oral route as per
decision of the treating neonatologist. IL was decided by Tochen’s
formula (weight in (kg) + 6cm) [15]. Birthweight or current weight,
whichever was higher, was used to estimate IL. After intubation, ET was
readjusted by auscultation and fixed at a position where air entry was
bilaterally equal. Neck position was maintained in slight extension with
the help of a shoulder roll during USG and radiograph. After intubation
and fixation of the ET, exact IL was calculated. The part of ET present
exterior to the lips (A) was measured from a visible centimeter mark on
the adapter end of ET to the corner of the lip with a paper tape
following curvature of the ET. Exact IL was calculated by subtracting
this length (A) from length of ET (B) up to that mark (Web. Fig 1).
X-ray was ordered and USG was done to determine ET tip position
following intubation. No change in ET position was done based on USG
findings until X-ray film was available.
ET tip position was determined by USG following
intubation using Sonosite M-Turbo portable ultrasound machine with phase
array probe of 8-4 MHz frequency. To minimize the variability of USG
measurements, only two of the investigators conducted all USG after
appropriate training.
Mid-sagittal views were obtained by placing the probe
on the infant’s lower neck and upper sternum in order to visualize the
ET. Warm gel was applied to the probe during USG. Care was taken to
ensure adequate oxygenation and temperature regulation throughout the
procedure. The bedside nurse was available to assist in calming the
infant. Arch of aorta was visualized by gray scale and color Doppler. ET
was identified as a linear echo dense structure. The ET tip was reliably
delineated by producing a minimal gentle movement with the help of an
assistant. Each image was zoomed and the distance of the ET tip from
superior border of arch of aorta was measured in the line of ET (Fig.
1). A total of three observations were made for each subject and
average of these measurements was taken. Both static images and video
clips were recorded and stored in the flashcard of USG machine to be
later transferred to the computer for storage. Twenty percent of the
videos were analyzed by a pediatric cardiologist for validation. Time
elapsed between end of intubation and completion of last measurement by
USG was recorded.
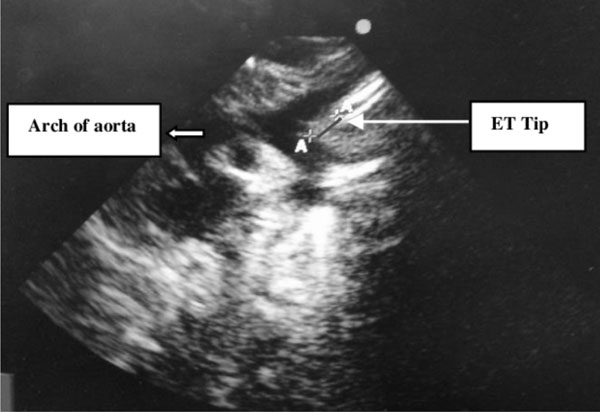 |
|
Fig. 1 Demonstrates linear echo
bright structure confirmed to be endotracheal tube (ET) by
gently moving the tube; AA distance of ET tip from arch of aorta
measured in the line of ET.
|
In 25 intubated infants, USG was done in succession
by both the investigators blinded to each other’s findings. A total of
three observations were made by each investigator for these infants.
Intraclass correlation coefficient (ICC) and Bland-Altman analysis were
used for measuring and testing the consistency, reliability and
agreement of USG measurements between the two investigators. A strong
intraclass correlation (ICC>0.9) was also observed between average USG
readings of both the investigators (ICC 0.98; 95% CI 0.96 to 0.99). A
strong intraclass correlation was also observed for all the three
measurements of the investigators (ICC 0.93; 95% CI 0.91, 0.95; and
0.97; 95% CI 0.95, 0.99). Bland-Altman analysis (Web Fig. 2)
showed mean difference of -0.02 mm (95% CI -0.05 to -0.01) in the
measurements of the two co-investigators.
Corrective measure to place the ET in optimum
position was taken by the treating neonatologist after availability of
X-ray film. Time elapsed between intubation and availability of
X-ray film was noted. All X-ray films were later reviewed
by a radiologist and ET was classified as optimum (ET tip located
between upper border of T1 and lower border of T2 vertebral body), low
(ET tip lying below lower border of T2 vertebral body) or high (ET tip
situated above upper border of T1 vertebral body) [16].
All anthropometric measurements were made by a single
investigator on the day of enrolment. OFC was recorded with a paper tape
placed posteriorly on external occipital protuberance and anteriorly
above supraorbital ridges. CHL was measured with the help of a length
board (Seca 210) with knee extended and foot perpendicular to the
ground. SL was measured from the suprasternal notch to the tip of the
xiphoid process. NTL was noted from the base of the nasal septum to the
tip of the tragus. A total of three readings were made for each
parameter and mean of these readings was calculated.
The primary outcome was to calculate the distance
between optimally placed ET tip and arch of aorta across different
weight and gestation by USG. Secondary outcome included correlation
between IL of optimally placed ET and anthropometric parameters such as
weight, OFC, CHL, NTL and SL.
A pilot study was conducted in 15 infants to
calculate the mean and standard deviation (SD) of the distance between
optimally placed ET tip from arch of aorta by USG. Among very low birth
weight infants (birth weight <1500 g), mean (SD) was found to be 0.30 cm
(0.11). Considering precision of 10% across the mean, sample size for
very low birth infants was found to be 52. Similarly, for infants
weighing >1500 g, mean (SD) was 0.60 cm (0.20) and considering a
precision of 10%, sample size was found to be 42. Therefore, a total of
94 infants with optimally placed ET were required to derive normative
data of the distance between optimally placed ET tip and arch of aorta
by USG.
Statistical analysis: Analysis of data was done
using SPSS software version 20.0. Chi square or Fisher’s exact test was
used to compare categorical variables. Student’s t test and Mann
Whitney test were applied to compare independent parametric and
non-parametric variables, respectively. Non-parametric related samples
were tested by Wilcoxon signed rank test. Two sided P value <0.05
was considered significant. Pearson’s correlation and linear regression
were used to analyze the relationship between anthropometric data
(weight, OFC, CHL, NTL and SL) and IL. IL of correctly placed ET was the
dependent variable and anthropometric parameters were independent
variables for the correlation and regression analysis.
The intraclass correlation coefficient (ICC) was used
to determine the consistency, reliability and reproducibility (inter and
intra observer variability between two observers) of USG measurements
across the two examiners. The corresponding limits of agreements were
calculated by means of Bland-Altman analysis after assuring the
normality of the differences between two sets of results (i.e.,
the paired observations of principal investigator and co-investigator),
which was examined using Kolmogorov–Smirnov test.
Results
A total of 1157 infants were admitted during the
study period. Consent was obtained for 496 infants at risk of
intubation, out of which 258 were intubated. During 68 intubations,
investigators were not available and an additional 57 infants were
excluded due to various other reasons (Fig. 2). A total of
133 infants were included, of which 101 had optimally placed ET on X-ray.
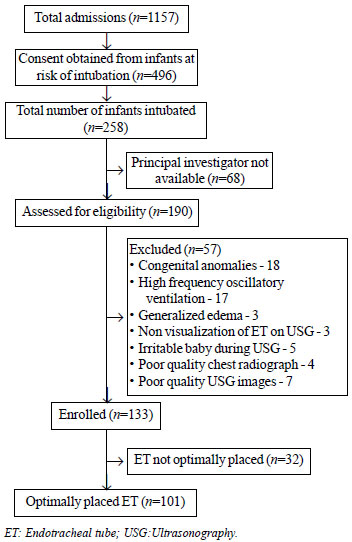 |
|
Fig. 2 Study flow chart.
|
The baseline characteristic of enrolled infants is
described in Table I. Mean (SD) IL and USG distance
between optimally placed ET tip and arch of aorta in different weight
and gestation groups is depicted in Table II. A total of
32 infants had malpositioned ET. Deep intubation was twice as common as
high intubation (15.10% (21/133) vs 8.30% (11/133); P=0.02).
Proportion of malpositioned ET in infants <1500 g was higher compared to
infants ³1500g
(31.40% (26/83) vs 12% (6/50); P=0.01). Similarly,
malposition was more common in infants of gestation <32 weeks compared
to ³32 weeks
(33.80% (23/68) vs 13.90% (9/65); P<0.01). The median
(IQR) time from intubation to completion of three readings of USG was
less than the time required for obtaining X-ray film (12.00
(8.00-15.00) min vs 98.00 (64.00-132.00) min; P<0.001).
TABLE I Baseline Characteristics of Overall Study Population (N=133)
|
Parameter |
n(%) |
|
Gestation in (wk)* |
30.8 (4.6)
|
|
Birth weight in (g)* |
|
|
<1500 |
992.7 (272.0) |
|
≥1500 |
2480.1 (597.3) |
|
PMA (wk)* |
32.0 (5.3) |
|
Weight at enrolment (g)* |
|
|
<1500 |
1028.9 (274.0) |
|
≥1500 |
2456.1 (597.3) |
|
Weight for gestation |
|
|
AGA |
100 (75.2) |
|
SGA |
26 (19.5)
|
|
LGA |
7 (5.3) |
|
Gender |
|
|
Male |
99 (74.4) |
|
Weight enrolment groups (g) |
|
|
<1000 |
45 (33.8) |
|
1000-1499 |
38 (28.6) |
|
1500-1999 |
16 (12.0) |
|
2000-2499 |
8 (6.0) |
|
≥2500
|
26 (19.5) |
|
PMA enrollment groups (wk) |
|
|
<28 |
31 (23.3) |
|
28-31 |
37 (27.8) |
|
32-35 |
25 (18.8) |
|
≥36 |
40 (30.1) |
|
Data expressed as n (%) or *mean (SD); PMA: Post menstrual age;
AGA: Appropriate for gestational age; SGA: Small for gestational
age; LGA: Large for gestational age. |
TABLE II Insertional Length and Normative Data of the Distance Between Optimally Placed ET Tip and
Arch of Aorta by USG Across Different Weight and Gestation Categories (N=101)
|
Categories |
Insertional
|
USG distance
|
|
length (cm) |
Mean (SD) |
95% CI |
|
Mean (SD) |
|
|
|
Weight (g) |
|
<1000 (n=30) |
5.80 (0.42) |
0.73 (0.21) |
0.65-0.80 |
|
1000-1499 (n=27) |
6.46 (0.46) |
0.86 (0.18) |
0.79-0.94 |
|
1500-1999 (n=14) |
6.97 (0.54) |
0.94 (0.29) |
0.77-1.12 |
|
2000-2499 (n=6) |
7.26 (0.44) |
0.98 (0.13) |
0.84-1.13 |
|
≥2500 (n=24) |
8.30 (0.54) |
1.10 (0.35) |
0.95-1.26 |
|
PMA Gestation (wk) |
|
<28 (n=20) |
5.83 (0.41) |
0.65(0.19) |
0.58-0.76 |
|
28-31 (n=25) |
6.20 (0.56) |
0.83 (0.15) |
0.77-0.90 |
|
32-35 (n=20) |
6.84 (0.58) |
0.94 (0.22) |
0.84-1.04 |
|
≥36 (n=36) |
7.78 (0.91) |
1.04 (0.34) |
0.93-1.16 |
|
ET: Endotracheal tube; PMA: Post menstrual age. |
TABLE III Distance Between Endotracheal Tube Tip and Arch of Aorta by Ultrasonography Across
Different Gestation and Weight (N=101)
|
Percentiles |
|
Parameter |
N
|
5th
|
10th
|
25th
|
50th
|
75th
|
90th
|
95th
|
|
Weight (g) |
|
|
|
|
|
|
|
|
|
<1000
|
30 |
0.30 |
0.42 |
0.60 |
0.72 |
0.88 |
1.04 |
1.08 |
|
1000-1499 |
27 |
0.49 |
0.62 |
0.75 |
0.92 |
0.96 |
1.08 |
1.22 |
|
1500-1999 |
14 |
0.47 |
0.55 |
0.75 |
0.92 |
1.09 |
1.49 |
- |
|
2000-2499 |
6 |
0.76 |
0.76 |
0.89 |
0.98 |
1.12 |
- |
- |
|
≥ 2500
|
24 |
0.50 |
0.58 |
0.76 |
1.12 |
1.39 |
1.61 |
1.75 |
|
Post-menstrual age (wk) |
|
|
|
|
|
|
|
|
|
<28 |
20 |
0.27 |
0.34 |
0.55 |
0.69 |
0.81 |
0.93 |
1.04 |
|
28-316/7 |
25 |
0.58 |
0.63 |
0.71 |
0.82 |
0.93 |
1.08 |
1.08 |
|
32-356/7 |
20 |
0.47 |
0.72 |
0.78 |
0.97 |
1.04 |
1.30 |
1.39 |
|
≥ 36
|
36 |
0.49 |
0.55 |
0.76 |
1.06 |
1.23 |
1.58 |
1.67 |
TABLE IV Pearson’s Correlation (r) and Linear Regression Equations for Insertional
Length and Various Anthropometric Measurements (N=101)
|
Parameter |
r |
P value |
Regression equation (R2) |
|
Weight (Kg) |
0.906 |
<0.001 |
Wt (Kg) + 4.95 |
|
OFC (cm) |
0.903 |
<0.001 |
0.223×OFC (cm)+0.49 |
|
NTL (cm) |
0.898 |
<0.001 |
0.822×NTL (cm)+1.24 |
|
CHL (cm) |
0.896 |
<0.001 |
0.154×CHL (cm)+0.57 |
|
STL (cm) |
0.860 |
<0.001 |
0.752×STL (cm)+2.26 |
CHL: Crown heel length, OFC: Occipito frontal circumference,
NTL: Nasal tragus length, STL: Sternal length, IL: Insertional
length. |
USG distance between ET tip and arch of aorta was
also compared in infants <1500g vs
³1500g and <32 weeks
vs ³32
weeks. Mean (SD) USG distance in VLBW population was significantly less
than the mean distance for infants with weight >1500g (0.78
(0.21) vs 1.04 (0.32); P<0.001). Similarly, mean (SD)
distance in infants with post menstrual age <32 wk was significantly
less as compared to the distance for the population
³32 weeks (0.77
(0.18) vs 1.01 (0.30); P<0.001). Table III
illustrates centiles of the ultrasound distance between ET tip and arch
of aorta across different weight and gestation groups. The degree of
correlation between IL and anthropometric parameters and the regression
equation to predict insertional length from weight, OFC, CHL, NTL and SL
have been described in Table IV. Linear
relationship between IL and various anthro-pometric parameters has been
displayed in the figure (Web Fig. 3a-3e).
Discussion
Endotracheal tube position can be confirmed by
bedside USG without exposing the infant to radiation and handling
[7,8,11,17]. Ultrasound
studies have revealed that a distance of 0.5 to 1 cm between ET tip and
arch of aorta suggests its correct placement [7,11]. However,
this distance is likely to differ across different weight and gestation
due to variation in tracheal length [12-14]. We conducted an
observational study with the primary objective to derive normative data
of the distance between optimally placed ET tip from arch of aorta by
USG across different weight and gestation.
In our study, we determined the ET position by USG in
mid-sagittal view and measured the distance between ET tip to arch of
aorta in the line of ET. Our method was similar to that described by
Slovis, et al. [7].
They observed that the distance of ET tip to carina on X ray and
ET tip to arch of aorta by USG had good degree of correlation. Sethi,
et al. [11], using a similar method, found that the distance between
ET tip to arch of aorta was 0.5–1 cm in 48 out of 53 correctly placed
ET. However, both the authors did not account for intra or
inter-observer variability during USG measurements.
Lingle [8] described a modified technique to
visualize the ET by using an USG ‘stand-off pad’ in 6 neonates, which
obviated the need to extend the neck and therefore reduce the risk of
tube displacement. This method was used only in six infants and lacks
validation. In two other studies, Dennington, et al. [10] and
Najib, et al. [18] measured the distance between ET tip to an
anatomic equivalent of carina (superior portion of the right pulmonary
artery) and found good correlation with radiograph [10,18].
Chowdhry, et al. [17] measured the distance
from the point of maximal curvature of the arch of aorta to the ET tip
by USG and a minimum distance of 1 cm was used to define "not deeply
placed ET". This distance was derived from preliminary analysis of
computed tomography scans of infants between zero to three months of
age. The study reported a concordance of 94.6% between USG and
radiograph [17]. However, in
none of these studies, authors reported variation in measurements across
different weight and gestation.
In our study, we found that the distance between
optimally placed ET tip from arch of aorta increases with increase in
weight and gestation. Anatomical studies can explain our results. In a
prospective study of routine autopsies which included 274 fetuses (15-41
weeks) and 26 infants (0 to 3 months), anatomical measurements of larynx
and trachea showed linear relationship between tracheal length and
gestational age, body weight and length [13]. In term infants, trachea
measures 5-6 cm, whereas in premature infants it can be as short as 3 cm
[19, 20]. Therefore, a
distance of 0.5-1 cm between ET tip and arch of aorta as suggested by
previous authors may not result in optimum placement of ET in all the
infants. Our study is in agreement with the biological plausibility of
variation in tracheal length and reports variation in the USG distance
of ET tip from arch of aorta in infants of various weight and gestation.
We also compared the time required for obtaining X-ray
film and USG. The mean (SD) time taken to conduct USG from the time of
intubation was less than the time required for availability of X
ray film. Lesser mean (SD) time taken to conduct USG as compared to
radiograph has previously been also reported [11].
The time required for radiograph may vary
depending on the setup, availability of bedside machine and technician,
and time required to develop and deliver the X-ray film to the
clinician. On the contrary, point of care USG and availability of
personnel at the bedside avoids unnecessary delay in confirmation of ET
tip position.
In clinical practice, IL is predicted based on
various anthropometric parameters [2-4]. In our study, IL
correlated strongly with anthropometric parameters (weight, CHL, OFC,
NTL and SL). NRP guidelines till 2010 recommended weight-based formula
given by Tochen (Wt in kg + 6 cm) for deciding IL [21-23]. However, we
found that the regression equation that best predicted IL for optimum
placement of ET is wt in kg + 4.95 cm.
Our findings suggest that in our population Tochen’s
formula overestimates IL by approximately 1 cm. In another study from
India, Tatwavedi, et al. [16] also showed similar relationship
between weight and IL [IL=weight in kg +5 (r=0.81, P<0.001)].
Similarity in Tatwavedi and our findings may be due to similar
population enrolled and the variation from other studies may be
attributed to racial difference in tracheal size [24-26].
Weight may not be available immediately after birth
or during emergencies and may be fallacious in infants who are edematous
or dehydrated. One of the easily measurable anthropometric parameter for
prediction of IL is NTL. It can be measured quickly as the two
landmarks, base of the nasal septum and tragus are well defined and
fixed. In addition, NTL measurement can be done without disturbing the
sick infant. As per the regression equation in our study, IL can be
predicted as 0.82 NTL (cm) +1.24. NRP 2015 guidelines also endorse use
of NTL to decide IL [27]. In our study, we found good correlation
between IL and other anthropometric parameters (SL, OFC and CHL).
However, their regression equations seem complicated, difficult to
memorize and use in clinical practice. We also observed that it was
difficult to measure SL in infants with marked chest retractions.
The mean and SD of the distance between ET tip and
arch of aorta calculated in the pilot study are different from the final
results. Considering the final results, sample size would have been
smaller; however due to paucity of literature, we were dependent on the
pilot study to calculate the required sample size. The limitation of our
study is that it only reports the normative data but it does not
validate what proportion of ET would be optimally placed by using this
data.
Our study reports the normative data of the distance
between optimally placed ET tip and arch of aorta by USG in neonates.
However, we emphasize that USG is a skill-based technique and competency
training is required before this normative data can be used in clinical
practice. In addition, we conclude that IL can be predicted based on
various anthropometric parameters such as weight, CHL, OFC, NTL and SL.
Contributors: AT: conceptualized and
designed the study, provided training to perform ultrasound, supervised
data collection, conducted statistical analysis and helped in manuscript
writing; PS, AT: performed ultrasounds, collected data and drafted
initial manuscript; NK, PG: study design, supervised the conduct of the
study and helped in manuscript writing; NA: was involved in planning the
study and analyzed and validated the videos of ultrasound. All authors
approved the final manuscript.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Ultrasound is a feasible tool to determine
endotracheal tube position and has good agreement with chest
radiograph.
What This Study Adds?
• This study provides normative data using
ultrasound for the distance between endotracheal tube tip and arch
of aorta across various weight and gestation groups.
|
References
1. da Silva O, Stevens D. Complications of airway
management in very-low-birth-weight infants. Biol Neonate. 1999;75:40-5.
2. Shukla HK, Hendricks-Munoz KD, Atakent Y, Rapaport
S. Rapid estimation of insertional length of endotracheal intubation in
newborn infants. J Pediatr. 1997;131:561-4.
3. Kempley ST, Moreiras JW, Petrone FL. Endotracheal
tube length for neonatal intubation. Resuscitation. 2008;77:369-73.
4. Embleton ND, Deshpande SA, Scott D, Wright C,
Milligan DW. Foot length, an accurate predictor of nasotracheal tube
length in neonates. Arch Dis Child Fetal Neonatal Ed. 2001;85:F60-4.
5. Mainie P, Carmichael A, McCullough S, Kempley ST.
Endotracheal tube position in infants requiring emergency interhospital
transfer. Am J Perinatol. 2006;23:121-4.
6. Poznanski AK, Kanellitsas C, Roloff DW, Borer RC.
Radiation exposure to personnel in a neonatal nursery. Pediatrics.
1974;54:139-41.
7. Slovis TL, Poland RL. Endotracheal tubes in
neonates: Sonographic positioning. Radiology. 1986;160:262-3.
8. Lingle PA. Sonographic verification of
endotracheal tube position in neonates: A modified technique. J Clin
Ultrasound. 1988;16:605-9.
9. Galicinao J, Bush AJ, Godambe SA. Use of bedside
ultrasonography for endotracheal tube placement in pediatric patients: A
feasibility study. Pediatrics. 2007;120:1297-303.
10. Dennington D, Vali P, Finer NN, Kim JH.
Ultrasound confirmation of endotracheal tube position in neonates.
Neonatology. 2012;102:185-9.
11. Sethi A, Nimbalkar A, Patel D, Kungwani A,
Nimbalkar S. Point of care ultrasonography for position of tip of
endotracheal tube in neonates. Indian Pediatr. 2014;51:119-21.
12. Wailoo MP, Emery JL. Normal growth and
development of the trachea. Thorax. 1982;37:584-7.
13. Fayoux P, Marciniak B, Devisme L, Storme L.
Prenatal and early postnatal morphogenesis and growth of human
laryngotracheal structures. J Anat. 2008;213:86-92.
14. Szpinda M, Daroszewski M, WoŸniak A, Szpinda A,
Mila-Kierzenkowska C. Tracheal dimensions in human fetuses: an
anatomical, digital and statistical study. Surg Radiol Anat.
2012;34:317-23.
15. Tochen ML. Orotracheal intubation in the newborn
infant: a method for determining depth of tube insertion. J Pediatr.
1979;95:1050-1.
16. Tatwavedi D, Nesargi SV, Shankar N, Rao S, Bhat
SR. Evaluation of body parameters for estimation of endotracheal tube
length in Indian infants. Eur J Pediatr. 2015;174:245-9.
17. Chowdhry R, Dangman B, Pinheiro JM. The
concordance of ultrasound technique versus X-ray to confirm
endotracheal tube position in infants. J Perinatol. 2015;35:481-4.
18. Najib K, Pishvs N, Amoozegar H, Pishdad P,
Fallahzadeh E. Ultrasonographic confirmation of endotracheal tube
position in neonates. Indian Pediatr. 2016;53:886-8.
19. Griscom NT, Wohl ME. Dimensions of the growing
trachea related to age and gender. AJR Am J Roentgenol. 1986;146:233-7.
20. Standring, S, editor. Gray’s Anatomy: The
Anatomical Basis of Clinical Practice. 41st ed. New York: Elsevier
Limited; 2016.
21. Guidelines 2000 for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care. Part 11: Neonatal Resuscitation. The
American Heart Association in collaboration with the International
Liaison Committee on Resuscitation. Circulation. 2000;102:I343-57.
22. American Heart Association. 2005 American Heart
Association (AHA) Guidelines for Cardiopulmonary Resuscitation (CPR) and
Emergency Cardiovascular care (ECC) of Pediatric and Neonatal Patients:
Pediatric basic life support. Pediatrics.2006;117:e989-1004.
23. Kattwinkel J, Perlman JM, Aziz K, Colby C,
Fairchild K, Gallagher J, et al. Part 15: Neonatal Resuscitation:
American Heart Association Guidelines for Cardiopulmonary Resuscitation
and Emergency Cardiovascular Care. Circulation. 2010;122:909-19.
24. Allen MS. Surgical anatomy of the trachea. Chest
Surg Clin N Am. 2003;13:191-9.
25. Mi W, Zhang C, Wang H, Cao J, Li C, Yang L, et
al. Measurement and analysis of the tracheobronchial tree in Chinese
population using computed tomography. PLoS One. 2015;10:e0123177.
26. Cinar U, Halezeroglu S, Okur E, Inanici MA,
Kayaoglu S. Tracheal length in adult human: The results of 100
autopsies. Int J Morphol. 2016;34:232-6.

