|
|
|
Indian Pediatr 2016;53: 619-622 |
 |
Profile and Trends
of Rotavirus Gastroenteritis in Under-five Children in India
(2012-2014): Preliminary Report of the Indian National Rotavirus
Surveillance Network
|
|
CP Girish Kumar, S Venkatasubramanian,
*Gagandeep Kang,
#Rashmi Arora and Sanjay
Mehendale, for the National Rotavirus Surveillance Network
From National Institute of Epidemiology, Chennai;
*Christian Medical College and Hospital, Vellore; and #Indian
Council of Medical Research, New Delhi; India
Correspondence to: Dr CP Girish Kumar, National
Institute of Epidemiology, II Main Road, TNHB, Ayapakkam, Chennai 600
077, India.
Email: [email protected]
Received: January 02, 2016;
Initial review: January 04, 2016;
Accepted: May 12, 2016.
|
Objective: To estimate the burden of rotavirus-associated
gastroenteritis in India.
Methods: Hospital-based surveillance network was
established, with clinical evaluation and laboratory testing for
rotavirus among children aged below 5 years hospitalized with acute
gastroenteritis.
Results: Between September 2012 and December
2014, stool samples from 10207 children were tested and rotavirus was
detected in 39.6% of cases. Infections were more commonly seen among
younger children (<2 years). Detection rates were higher during cooler
months of September – February. Among rotavirus infected-children, 64.0%
had severe or very severe disease. G1P[8] was the predominant rotavirus
genotype (62.7%) observed during the surveillance period.
Conclusions: Surveillance data highlights the
high rotavirus disease burden and emphasizes the need for close
monitoring to reduce morbidity and mortality associated with rotavirus
gastroenteritis in India.
Key words: Diarrhea, Epidemiology, Prevalence, Trends.
|
|
Rotavirus is the leading cause of severe childhood
gastroenteritis/diarrhea worldwide, and is estimated to account for
about one-third of deaths attributable to diarrhea in children under
five years of age [1,2]. In this report, we present the findings of the
rotavirus surveillance carried out as part of the National Rotavirus
Surveillance Network (NRSN) established by the Indian Council of Medical
Research (ICMR). This preliminary report describes rotavirus burden in
children admitted with acute gastroenteritis between September 2012 and
December 2014 by age, region, seasons and also the diarrheal disease
severity pattern.
Methods
The NRSN surveillance protocol was developed based on
a modification of the WHO generic protocol for rotavirus surveillance
[3]. Prospective surveillance for diarrhea-related hospitalizations
among children under five years of age was carried out in 28
hospital-based surveillance units spread across 17 states and two union
territories (UT) of India (Details of study sites are provided in a
companion paper in this issue) [4], and in
Web Annexure 1.
The surveillance units or clinical recruitment sites (CRS) were linked
with either Referral or Regional Laboratories. These clinical
recruitment sites were either governmental (n=16) or private (n=12)
health care facilities for pediatric patients. The study was initiated
after obtaining approvals by the institutional ethics committees of the
National Institute of Epidemiology (NIE), all the participating
reference and referral laboratories, and the CRS.
All children aged
£59 months who
presented to a participating CRS with acute gastroenteritis (³3
loose stools in a 24 hour period for 5 or less days), and required
hospitalization for diarrhea management, were enrolled after obtaining
informed and written consent from the accompanying parent or guardian.
Study medical officers enrolled eligible children and collected clinical
and demographic details on standardized clinical recruitment forms
(CRF).
Whole stool specimens (~5 mL) were collected and
transported within 2 hours to the testing laboratory or stored in a
refrigerator at 4 0C until
transportation. Samples stored at 40C
were transported in boxes with ice packs at weekly or fortnightly
intervals to the testing laboratories. All stool samples were subjected
to rotavirus screening using commercial enzyme immunoassay (Premier
Rotaclone, Meridian Biosciences) kits following
the manufacturer’s instructions. Rotavirus positive samples were
subjected to rotavirus genotyping for VP7 (G-typing) and VP4 (P-typing)
by Reverse-transcription polymerase chain reaction (RT-PCR) [5,6].
Initially all positives were genotyped but subsequently it was decided
to restrict genotyping to every third positive sample at each testing
laboratory. There were no gender, age, region-wise or seasonal
considerations for choosing samples for genotyping. Aliquots of all
samples were stored at -700C.
Data captured on the CRF were entered at each of the
regional and referral laboratories using the online data capture portal
hosted on the NIE web server. Site- specific summary data on diarrheal
hospital admissions and rotavirus positivity data from laboratories was
received at the Coordination Center (NIE) on a monthly basis.
Statistical analysis: Data were analyzed to
assess the proportions of rotavirus-positive cases in terms of
demographic factors, symptoms, disease severity (Vesikari score), median
duration of hospitalization, genotype status and also by season and
regions. Proportion ratios (PR) were calculated to compare the strength
of association of severe infection in rotavirus infected and uninfected
children with seasonal rotavirus burden and length of hospitalization.
Analyses were carried out using MS Excel 2007, SPSS v. 17.0 and
Stata v 10.0.
Results
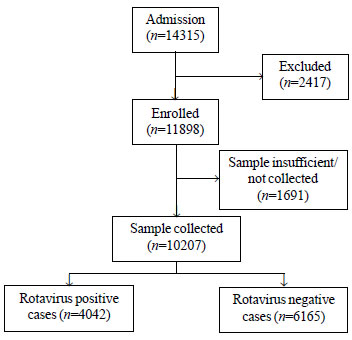
Details of case enrolment and testing are provided in
Fig. 1. Rotavirus was detected in 39.6% (4042/10207) of
diarrheal cases. Rotavirus infection was detected throughout the year in
all CRS. The detection rates were higher during December- February
(56.4%; 1668/2959).
 |
|
Fig. 1 Flow diagram summarizing
case enrollment and laboratory testing.
|
Most cases of rotavirus infection (41.5%; 3404/8206)
occurred among children less than 2 years of age (Table I).
The highest positivity (46.7%) was observed among children between 12
and 23 months of age. Among infants aged <3 months and <6 months, the
proportion of rotavirus positivity was 17.4% (127/729) and 27.4%
(497/1814) respectively. There were 118 neonates, and 22.9% (27/118) of
them were rotavirus positive. The median (IQR) age for rotavirus
infection was 12 (8,18) months, which was not significantly different
from that seen among cases of non-rotavirus gastroenteritis [median 12
mo. IQR (6,21) mo, P=0.51]. Hospital admissions due to rotavirus
gastroenteritis among boys (2520/6363) outnumbered that among girls
(1522/3844) with no significant difference in rotavirus positivity rates
between the two genders (39.6% vs 39.6%; P=0.992).
Burden of rotavirus varied significantly across
regions and seasons (Table I). Rotavirus infections
usually occurred more commonly during the cooler months of December -
February (56.4%), followed by September -November (38.4%).
TABLE I Rotavirus Positivity in Children Hospitalized with Acute Gastroenteritis
|
Variables |
Levels |
N |
Rotavirus |
|
|
|
positivity, n (%) |
|
Age (mo) |
0-2 |
729 |
127 (17.4) |
|
3-5 |
1085 |
370 (34.1) |
|
6-11 |
3148 |
1393 (44.3) |
|
12-23 |
3244 |
1514 (46.7) |
|
≥24 |
2001 |
638 (31.9) |
|
Median (IQR) |
|
12 (8 – 18) |
|
Gender |
Male |
6363 |
2520 (39.6) |
|
Female |
3844 |
1522 (39.6) |
|
Disease |
Mild [0-5] |
433 |
123 (28.4) |
|
severity |
Moderate [6-10] |
3779 |
1334 (35.3) |
|
Severe [11-15] |
5509 |
2394 (43.5) |
|
Very Severe [16-20] |
484 |
191 (39.5) |
|
Treatment |
Oral |
1987 |
788 (39.7) |
|
Intravenous |
8220 |
3254 (39.6) |
|
Season |
Dec-Feb |
2959 |
1668 (56.4) |
|
Mar-May |
1838 |
694 (37.8) |
|
Jun-Aug |
2239 |
461 (20.6) |
|
Sep-Nov |
3171 |
1219 (38.4) |
|
Region |
East |
2032 |
827 (40.7) |
|
West |
1755 |
689 (39.3) |
|
South |
4080 |
1521 (37.3) |
|
North |
2340 |
1005 (42.9) |
|
Oral RV vaccination |
Yes |
340 |
111 (32.6) |
Analysis of diarrheal disease severity showed that
the proportion rotavirus positive (64%) was greater among children with
very severe or severe disease compared to children with mild to moderate
infection (36%). A proportion ratio analysis revealed that proportion of
severe rotavirus gastroenteritis was more during December-February
(ratio: 1.75; CI: 1.65- 1.85), and these children were likely to stay in
the hospital for ³3
days (ratio: 1.72; CI: 1.60-1.85) (Table II).
TABLE II Proportion Ratio Analysis
|
A. |
Analysis of prevalence of severe gastroenteritis across seasons
|
|
Season |
Rotavirus |
Rotavirus |
PR (95 % CI) |
|
positive |
negative |
|
|
(n=2585) |
(n=3408) |
|
|
Dec -Feb |
1103 |
687 |
1.748 (1.654, 1.846) |
|
Mar-May |
400 |
609 |
0.904 (0.833, 0.982) |
|
Jun-Aug |
282 |
1029 |
0.437 (0.393, 0.487) |
|
Sep-Nov |
800 |
1083 |
0.978 (0.918, 1.042) |
|
B. |
Analysis of length of hospitalization (≥3
days) among cases of severe gastroenteritis across seasons
|
|
Season |
Rotavirus |
Rotavirus |
PR (95 % CI) |
|
positive |
negative |
|
|
(n=1569) |
(n=2121) |
|
|
Dec-Feb |
651 |
425 |
1.723 (1.605, 1.850) |
|
Mar-May |
268 |
396 |
0.939 (0.848, 1.039) |
|
Jun-Aug |
172 |
647 |
0.432 (0.376, 0.495) |
|
Sep-Nov |
478 |
653 |
0.991 (0.914, 1.076) |
|
*Proportion Ratio (PR) = Proportion of RV +ve cases in a
particular season / Proportion of RV +ve cases in remaining
seasons. |
Only 3.3% (340/10206) of enrolled children had a
history of rotavirus vaccination, and among them 111 children (32.6%)
were rotavirus positive. Majority of vaccinated children were seen at
private hospitals (87.9 %).
During the reporting period, 15 (0.15%) enrolled
children died during their hospital stay. Rotavirus antigen was detected
in stool from four children. Twelve children were below one year of age
with a median (IQR) age of 5 (1,7) months. On admission, nine children
had severe to very severe diarrhea (median (IQR) Vesikari score 15
(14,15)). Thirteen children with severe diarrheal disease (median (IQR))
Vesikari score 14 (10,15) had received intravenous fluids and the
remaining two children with mild to moderate diarrhea had received oral
rehydration. Analysis of the cause of death revealed that the children
had died due to serious complications viz. sepsis and shock (10
cases), sepsis and meningitis (3 cases), bronchopneumonia (1 case), and
milk-aspiration (1 case).
Analysis of overall distribution of various rotavirus
genotypes showed the preponderance of G1P[8] strains (62.7%) followed by
G2P[4] strains (7.6%) (data not shown). Among neonates hospitalized with
gastroenteritis, eight genotypes were observed with G1P[8] (45.5%;
10/22) as the commonest strain.
Discussion
Using a standardized approach for patient enrollment
and testing in country-wide surveillance, rotavirus was detected in
39.6% of children admitted with diarrhea. This is consistent with
findings from the earlier phase of rotavirus surveillance in India
[4-6]. Our report highlights the substantial rotavirus disease burden in
India. In the present round of surveillance, although most cases of
rotavirus gastroenteritis were seen among children between 12 and 23
months of age, it was documented in all age sub-groups, including
neonates. These rates are; however, higher than previously reported from
India [5-7]. Neonatal infections which are usually mild or asymptomatic
are caused by different nursery rotavirus strains, but in this study,
nearly half the neonates (10/22 genotyped) had gastroenteritis due to
G1P[8], which was also the predominant genotype circulating among older
children. These findings are in agreement with data from the previous
surveillance that reported early incidence of rotavirus infection in
India [6].
Analysis of diarrheal disease severity showed that
children with rotavirus infection have severe disease and the occurrence
of severe rotavirus gastroenteritis was more commonly observed during
December – February period that represents cooler season in India. The
seasonal pattern observed in this surveillance period, with more
infections occurring during cooler months was similar to the observation
during the previous iteration of the NRSN, and is consistent with
reports from most parts of the world [6,8,9].
The reported low mortality among hospitalized
children with acute gastroenteritis could not be attributed to rotavirus
infection. This probably reflects that an effective diarrheal disease
management protocol is practiced in the health care facilities
participating in this surveillance. It would be necessary to study the
community burden of rotavirus gastroenteritis and related morbidity and
mortality outcomes to know the true burden in the community settings and
non-hospitalized children. The limitation of the present analysis is
that the findings that have been presented in this paper represent
interim analysis of data.
The data from the expanded NRSN was already available
with policy makers before the recent decision to introduce rotavirus
vaccine in the UIP [10]. Continued surveillance and studies among
vaccinated children will generate evidence regarding impact of rotavirus
vaccine rolled out through the national program on morbidity and
mortality due to rotavirus infections in young Indian children.
The successfully implemented NRSN surveillance
platform will continue to generate data on trends in rotavirus disease
burden and its correlates. It will also contribute significantly in the
assessment of the impact of the rotavirus vaccine after implementation.
Acknowledgements: M.Chiranjeevi, Technical
Assistant, NRSN project Team at NIE for support in statistical analysis.
Contributors: CPGK, SMM: Network coordination,
conceptualization and manuscript writing; SV: Contributed to network
coordination, statistical analysis and manuscript writing; GK:
Coordinated laboratory activities in the network and provided
intellectual inputs for manuscript development; RA: Coordination at ICMR
level and provided intellectual inputs to for manuscript development.
All authors approved the final manuscript.
Funding: Indian Council of Medical
Research; Competing Interests: None stated.
|
What This Study Adds?
•
Rotavirus is associated with 40% of diarrheal episodes
requiring hospitalization in under-five children.
|
References
1. World Health Organization. Rotavirus Mortality
Estimates. Available from:
http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en.
Accessed March 21, 2016.
2. John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK,
Kang G. Rotavirus gastroenteritis in India, 2011-2013: Revised estimates
of disease burden and potential impact of vaccines. Vaccine.
2014;32:A5-9.
3. World Health Organization. Generic protocols (i)
hospital-based surveillance to estimate the Burden of Rotavirus
Gastroenteritis in Children and (ii) a Community Based Survey on
Utilization of Health Care Services for Gastroenteritis in
Children. Geneva. 2002.
4. Mehendale S, Venkatasubramanian S, Girish Kumar
CP, Kang G, Gupte MD, Arora R. Expanded Indian National Rotavirus
Surveillance Network in the context of rotavirus vaccine introduction.
Indian Pediatr.2016;53:575-81.
5. Kang G, Desai R, Arora R, Chitambar S, Naik TN,
Krishnan T, et al. Diversity of circulating rotavirus strains in
children hospitalized with diarrhea in India, 2005–2009. Vaccine.
2013;31:2879-83.
6. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance
of rotavirus disease and strains among Indian children aged <5 years. J
Infect Dis. 2009;200:S147–S53.
7. Saluja T, Sharma SD, Gupta M, Kundu R, Kar S,
Dutta A, et al. A multicenter prospective hospital-based
surveillance to estimate the burden of rotavirus gastroenteritis in
children less than five years of age in India. Vaccine. 2014;32:A13-9.
8. Brandt CD, Kim HW, Rodriguez WJ, Arrobio JO,
Jeffries BC, Parrott RH. Rotavirus gastroenteritis and weather. J Clin
Microbiol. 1982;16:478-82.
9. Jagai JS, Sarkar R, Castronovo D, Kattula D,
McEntee J, Ward H, et al. Seasonality of rotavirus in South Asia:
A meta-analysis approach assessing associations with temperature,
precipitation, and vegetation index. PLoS ONE. 2012;7:e38168.
10. Ministry of Health and Family Welfare Notable
Achievements and Initiatives- 2015. Press Information Bureau. Available
from: http://pib.nic.in/newsite/Print Release.aspx?relid=133853.
Accessed March 21, 2016.
|
|
|
 |
|

