|
Arvind Bagga
Pankaj Hari
From the Division of Pediatric Nephrology, Department of Pediatrics, All India Institute of Medical Sciences, New Delhi 110 029, India.
Reprint requests: Dr. Arvind Bagga, Department of Pediatrics, All India Institute
of Medical Sciences, Ansari Nagar, New Delhi
110 029,
India.
Vesicoureteric reflux (VUR) is the abnormal retrograde flow of urine from the bladder into the upper urinary tract. Primary VUR is a common congenital anomaly of the vesicoureteric
junction. Secondary VUR results from an anatomic or functional obstruction at any level in the urinary tract (e.g.,
posterior urethral valves, bladder neck obstruction, ureteral duplications, ureteroceles). VUR may also be present in 30-50% cases of meningomyelocele, prune belly syndrome and anorectal malformations.
In the presence of VUR the rise in intravesical pressure occurring during micturition is freely transmitted to the ureter and pelvis, and in severe cases into the papillary collecting duct and tubules. Reflux into the renal parenchyma is called intrarenal reflux (IRR). IRR is believed to cause renal scarring by allowing pathogenic organisms from the bladder to gain access to the renal parenchyma. The renal scarring associated with VUR is referred to
'
as reflux nephropathy (RN).
VUR is seen in a number of children with urinary tract infections (UTI). The presence of moderate to severe reflux is an important risk factor for acute pyelonephritis and subsequent RN. The late sequelae of RN are well recognized and include hypertension, proteinuria and chronic renal failure. These complications may often not present until the second or third decade of life. It is this potential for significant morbidity that has led to the current emphasis on early diagnosis, prompt antibiotic therapy, and early evaluation of the urinary tract of infants and young children with documented UTI.
Pathogenesis of VUR
Normally the ureters enter the muscular layer of the bladder obliquely and pursue a submucosal course. These intravesical segments of the ureters collapse during detrusor contraction, and serve as a valvular mechanism preventing
VUR. The effectiveness of this antireflux mechanism is directly related to the length of the sub- mucosal ureter(1,2).
Development of the ipsilateral ureter and kidney is mediated by the ureteral bud that arises from the Wolffian duct. A deviation in the site of origin of the ureteral bud can lead to an anomalous drainage system. For example, VUR may occur if the bud originates from the most caudal end of the duct, too close to the bladder. With the rotational incorporation of the Wolffian duct and ureter into the developing bladder, the ureteric orifice in this case lies lateral to and cephalad to its normal position in the developed bladder. This results in congenital shortening of the submucosal ureteral segment, resultant valve dysfunction and
VUR. The abnormal position of the ureteric bud also results in poor induction of the development of the kidney (renal dysplasia). The site of origin of the ureteral bud is most likely genetically determined(2).
The submucosal segment of the ureter lengthens with somatic growth, increasing the competence or the valve mechanism. Reflux, therefore, tends to diminish or disappear
with age. On the other hand, presence of UTI may transiently increase the severity of pre-existing VUR.
Prevalence
VUR is unusual in healthy children with an estimated prevalence in newborns of 0.4% to 1.8%(2,3). However, 30-50% children and 40-50% of infants with UTI have VUR(4). The condition is more prevalent in girls except during infancy when it is more common in boys(2,5). Thirty to 60% of cases with VUR develop RN, .with 5-10% progressing to end stage renal failure(6,7).
Reflux Nephropathy
RN refers to focal or diffuse areas of irreversible renal scarring associated with VUR. The renal scars are wedge shaped with cortical thinning and distortion of the underlying calyces. They are asymmetrically distributed, being more common at the poles. The Ask-Upmark kidney, previously
regarded as segmental renal hypoplasia, is now believed to be a form of RN.
Renal scarring in a patient with VUR may result from: (i) IRR of infected urine with interstitial inflammation and damage; (ii) high-pressure sterile reflux that may damage the kidney through a mechanical or immunologic mechanism; and (iii) abnormal embryological development with renaldysplasia. UTI is the most important risk factor for renal scarring. Other risk factors include younger age, recurrent UTI and delay in starting treatment of UTI.
High pressure sterile reflux, as seen in patients with posterior urethral valves and neurogenic bladder, may occasionally result in renal scars(6).
Thus, RN is an acquired condition that requires early detection if sequelae are to be prevented. Most renal scarring occurs early in life before the age of 3-5 years(2,8). However, new scars may be acquired at older ages as well(9). The incidence of scarring is related to the severity of VUR. By use of the International Classification, RN was seen in 85% patients with grade V, 37-64% with grade IV, 25% with grade III, and 6-14% with grade II VUR(10).
Consequences of Reflux Nephropathy
RN is the most common cause of unilateral renal disease in children. It is an important cause of sustained hypertension in children. Twenty-38% patients with RN show hypertension on follow-up(2); the risk increasing with the severity of renal scarring. The presence of VUR and RN may lead to complications during pregnancy, especially if associated with impairment of renal function. Patients with persistent reflux show a higher incidence of bacteriuria and pyelonephritis during pregnancy. There is also an increased risk for hypertension and
preeclampsia(1).
Even when VUR ceases (following surgery or spontaneously), and obstruction and recurrent UTI are no longer present, renal injury may continue. Several mechanisms have been proposed to explain such a process. Hyperfiltration injury in the unscarred segments of the kidney may result in progressive glomerulosclerosis. Systemic hypertension and immunologic injury may also contribute to progressive renal disease. RN is an important cause of end stage renal failure, contributing 10- 20% children entering renal replacement programs(10). In a study of adults and
children, 80% patients with RN who progressed to renal insufficiency had hypertension(12). Proteinuria and hypertension are important indicators of progressive renal damage and poor outcome.
A 27-year-follow-up of 30 patients with a focal renal scar, showed hypertension in 25% and end stage renal failure in 10%(13). The consequences of a post-pyelonephritic scar are often obscured because 3-4 decades may elapse between the first UTI and detection of hypertension and end stage renal disease.
Clinical Presentation
UTI is the commonest clinical presentation of VUR. Patients may however present in several other ways (Table I). All Children with UTI should be evaluated for VUR after the first infection has been treated successfully. VUR is also diagnosed with increasing frequency in infants, typically during postnatal investigation of antenatal hydronephrosis, and while screening sib- lings of cases with VUR.
RN is most frequently detected following recognition of UTI and VUR. A significant proportion may, in our country, present with features of hypertension and chronic renal failure. Rarely, a patient may develop secondary nephrotic syndrome.
.
TABLE 1
Clinical Presentations of Vesicoureteric Reflux and Reflux Nephropathy.
|
Vesicoureteric reflux |
Reflux nephropathy |
| Urinary tract
infection |
Urinary tract
infection |
| Fetal hydronephrosis |
Hypertension |
Associated
urinary
tract anomalies |
Chronic renal failure
|
| Screening of siblings and offsprings Urinary calculi |
Associated urinary tract anomalies |
Diagnosis
VUR is diagnosed on radiocontrast micturiting cystourethrography (MCU; Fig. 1) or radionuclidecystography (DRCG; Fig. 2). The suggested interval between the UTI and cystourethrography is 4-6 weeks or until voiding symptoms associated with UTI have resolved. MCU
is preferred as the initial examination in both sexes since it
enables grading of VUR (Fig. 3). In addition, it visualizes the urethra in boys and detects structural anomalies such as posterior urethral valves, ureteroceles, and bladder diverticula. However, the procedure is invasive, uncomfortable and involves considerable radiation exposure. Catheterization of the urinary tract also carries the risk of introducing bacteria at the time of the procedure. We therefore recommend that patients undergoing MCU should
receive cotrimoxazole in full dosage for 48 h from the time of the procedure.
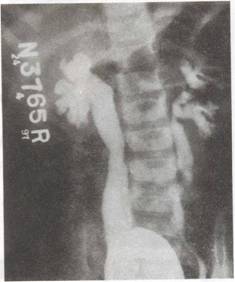 |
|
Fig. 1. Micturiting cystourethrogram (MCW showing bilateral VUR, grade IV on right and grade III on left-side. There is bilateral ureteral
and pelvic dilation with blunting of fornices in the right kidney. |
 |
|
Fig 2. 99m Tc-phylate
DRCG (direct radionuclide cystourethrography) study in a
2-year-old girl with recurrent urinary tract infections
showing gross VUR in right side. |
 |
|
Fig.
3. Grading of VUR (International Reflux Study Committee).
Grade
I: reflux up the ureter only; grade
II: reflux up the ureter, pelvis and calyces
with no dilation and normal calyceal fornices; grade
III; grade II VUR with mild
or moderate dilation and tortuosity of the ureter, no
blunting of calyces; grade IV: moderate dilation of ureter,
calyces and pelvis with complete blunting of fornices; grade
V: gross dilation of the ureter, pelvis and calyces with
absent papillary impressions
in the calyces(10). |
DRCG is more sensitive than MCU for diagnosis of VVR(14). It affords significantly lower radiation as compared to MCU, which makes it ideal for follow-up. Its important limitations are the inability to precisely grade the reflux and evaluate the urethra during voiding. The role of cystoscopic evaluation of the bladder in the diagnosis or follow-up of patients with VUR is limited.
Investigations to show RN include ultrasonography (US), intravenous pyelography (IVP), and radionuclide scanning. While US is freely available and non-invasive, it is highly operator-dependent, and does not provide any information on renal function. It also is relatively less sensitive in detecting renal scars in children under 5 years of age.
IVP is the traditional imaging technique for demonstrating RN. Fqcilities for performing this procedure are available in most hospitals in the country. The renal scars are characterized by thinning of the renal cortex with distortion of the under- lying calyx. The scars are most frequently seen in the upper or lower renal poles. In more severe forms, the renal damage is diffuse, with generalized reduction of renal mass, and uniform papillary changes. The main limitations of the IVP are its inability to provide significant information on renal function and failure to show early scars. IVP should be performed with caution in patients with impaired renal functions.
Renal cortical scintigraphy using technetium 99m-dimercaptosuccinic acid (DMSA) or glucoheptonate (GHA) is an excellent means for imaging the upper urinary tract. DMSA renal scintigraphy is the most sensitive technique for detecting focal renal abnormalities(15). Based on image morphology, experts can differentiate between acute pyelonephritis and permanent renal scars. The former is seen as an area of decreased uptake of DMSA with preservation of the normal .renal con- tour. Renal scars, on the other hand, are characterized by contraction and loss of functioning renal cortex (Fig. 4). Renal scans using GHA provide almost similar information.
Renal cortical scans are therefore useful: (i) for the diagnosis of acute pyelonephritis and RN; and (ii) to follow anatomically the progression of renal scarring and hypertrophy of the normal regions of the parenchyma. Facilities for renal scinti-graphy are however not easily available in India.
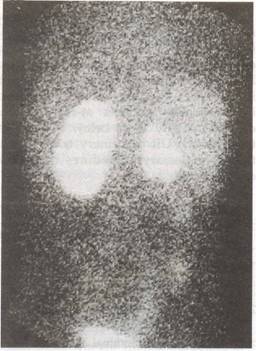 |
|
Fig. 4. 99m Tc-GHA (glucolzeptonate) scali (poste- rior view) of the patient in Fig. 2. Scan shows small right kidney with irregular contour consistent with scarring. The left kidney has a normal contour. Calculated function, 75% on the left kidney and 25% on the right kidney. |
Evaluation Following First Episode of UTI
The evaluation of patients with UTI is a topic of considerable importance and controversy. Radiologic evaluation now includes some combinations of. X-ray and abdominal ultrasound, MCV, renal scintiscan, and rarely IVP. Recommendations for evaluation of these children are not uniform and vary in different centers(2). Sound, prospective studies are still needed to assess the best indications and optimum imaging procedures in these children.
Antibiotic prophylaxis (see below) should be started in all patients, especially infants and young children, pending completion of investigations. Table II outlines our current protocol following the initial UTI.
The aim of the evaluation is to identify patients at-risk of
renal
damage, chiefly those below 5 years and those with VUR or urinary tract obstruction. Unnecessary procedures must how- ever be avoided.
TABLE
II
Evaluation Following the First UTI*
|
Age |
Initial investigations** |
| < 2 years |
Abdominal X-ray, ultrasound (US), and micturiting cystourethrogram (MCU) |
| 2-5 years |
Abdominal X-ray, US and DMSA renal scan (or IVP)
|
| > 5 years |
Abdominal X-ray
and US |
* Children with recurrent UTI
should be examined initially with X-ray, US and MCU regardless of age..
** Patients having abnormalities on initial evaluation should be investigated in detail
(see
text).
In children below the age of 2 years, a
plain abdominal radiograph, US and MCU are recommended. (Abdominal X-ray will show spina bifida and small renal calculi). These investigations will detect most cases at-risk of RN in this age group. A DMSA or GHA renal scan is performed if either of the above tests are abnormal. Between the ages of 2-5 years, a MCU is not immediately required. A US and DMSA scan is done in these patients. If facilities for a nuclear scan are not available, an IVP may be performed. If either the US or renal scan or IVP is abnormal, a MCU is necessary. By pursuing this policy the number of MCUs is this age group will be restricted. Children over the age of 5 years can be reliably screened with a plain abdominal radiograph and US. Further
,
imaging with MCU and GHA scan is done if the radiograph or US show abnormal findings.
An aggressive approach performing US, DMSA scans and MCU in all children below the age of 5 years, following their. first UTI,
has also been recommended by some experts.
Children requiring hospitalization for febrile infections should be screened for obstruction with US before discharge. The MCU may be delayed 4-6 weeks following the UTI to avoid demonstrating transient mild reflux secondary to inflammatory changes of the ureterovesical junction. It is however rare for reflux to be detected during UTI and then to disappear following treatment(16).
Evaluation in Children with Recurrent UTI
Children with more than one episode of UTI, irrespective of age, should be evaluated with US and MCU. A GHA renal scan is performed if either of the above tests are abnormal.
When hydronephrosis in the absence of VUR is seen on US, further evaluation with diuretic renography using Tc-Iabelled diethylenetriamine-penhiacetic acid (DTP A) or mercaptoacetyl-glycine (MAG-3) provides quantitative assessment of renal function and drainage of the dilated collecting system. Evaluation of these parameters differentiates nonobstructive-dilation from true obstruction of the urinary tract.
Antenatally Detected VUR
About 10-20% neonates with antenatally detected hydronephrosis are shown to have VUR after birth(17). The reflux is usually of high grade with a marked male predominance(5). Newborns who had been identified antenatally as having fetal hydronephrosis are placed on cephlexin prophylaxis at birth and evaluated by US and MCU after 1-2 weeks of life. DTPA
renal scan is done to assess total and differential renal function and exclude obstruction in the upper urinary' tract. In-utero VUR may lead to fetal nephropathy resulting in renal dysplasia and growth arrest; these kidneys are smooth and small with reduced renal function on cortical scintigraphy(18).
Familial VUR
There is evidence that primary VUR may be inherited as an autosomal dominant disorder(19). Screening studies show that one-third. of asymptomatic siblings and children of index cases have reflux on MCU(20). Children younger than 2 years have the highest incidence, which declines with increasing age; major sequalae of RN are rare.
Screening for VUR is recommended in siblings and offsprings of known refluxers. All asymptomatic siblings and offsprings must be screened with a renal US; those
showing abnormalities are .evaluated with a MCU or DRCG. Some nephrologists propose aggressive screening with US and DRCG in children under 2 years of age(20). DRCG may be preferred to MCU
in screening for reflux, since there is little reason to suspect an obstructive anomaly in these children.
Bladder Dysfunction and VUR
Urodynamicstudies are not recom- mended in most patients with VUR. How- ever, a significant proportion of children with detrusor-sphincter dyssynergia may show reflux(5). Patients with VUR
and symptoms of infrequent voiding, 'holding on', crouching,
daytime urgency, curtseying and incontinence should therefore be evaluated for evidence of bladder dysfunction(5).
Outcome of VUR
Though many variables determine the resolution rate, primary VUR shows a tendency to improve by the age of 6-10 years(4,21-24). Refluxing kidneys grow normally provided no recurrent UTI develops.
The severity of VUR is the most important factor that determines its resolution. As the grade of VUR
increases, the rate of its spontaneous resolution diminishes.
The spontaneous resolution rates are 85-92% in grade I, 63-76%
in grade II, 53-62% in grade ill, 32-33% in grade IV, and less
than 10% in grade V(21,22). Moreover the higher the grade of
reflux, the longer it takes to resolve. The
mean duration of VUR in patients with higher grades is substantially longer than in those with lower grades(22). Therefore, persistence of VUR beyond 12-18 months is not regarded an indication for surgical correction.
Management of VUR
The main objective of treatment is to prevent development of RN. The risk of renal scarring is highest in young children; new scars usually do not develop in children with normal renal scans at 4-5 years of age(23).
UTI must be recognized promptly, confirmed by culture, and treated aggressively. Elimination of bacteriuria should be documented on follow-up culture of urine 48-72 h after initiation of treatment. Children with UTI must be investigated promptly to detect VUR or other congenital anomalies
(Table
II).
Young children, particularly be- low 3 years of age, should be started on prophylactic antibiotics untill imaging studies are completed.
Long term prophylaxis with antibiotics or surgical repair are the chief options available for treating children with VUR.
Antibiotic Prophylaxis
Antibiotic prophylaxis to maintain sterile urine while awaiting spontaneous resolution is the cornerstone of treatment(2,3). The rationale of treatment is to reduce colonization, chiefly Enterobacteriaceae, at the urethral orifice thus reducing the incidence of ascending infection. Antibiotic prophylaxis is recommended for most patients, provided the urine is kept sterile and reflux is not complicated by obstruction. The antibiotic
used should have a broad spectrum of action and achieve a high
urinary concentration with minimal alteration of the bowel flora.
Co-trimoxazole, or nitrofurantoin may be administered as a single bed time dose (Table III). In view of nausea, vomiting and abdominal discomfort associated with the latter, co-trimoxazole is often preferred as the initial drug. Trimethoprim alone (in the same dose) is as effective as co-trimoxazole but is not available in India. Trimethoprim
has a slow excretion rate, and is excreted in prostate or vaginal secretions, thus preventing periurethral contamination. Nitrofurantoin
is however rapidly excreted in urine with no antibacterial
activity remaining after 8 to 12 h. Thus failure to take nitrofurantoin
for 1-2 days puts the patients at risk for UTI. Many centers prefer to use cephalexin in infants less than 4-6 months old; it is also a useful drug in children with G6PD deficiency. Drugs that tend to select resistant bacteria in the bowel including amoxicillin, nalidixic acid and other quinolones are not suitable for chemoprophylaxis.
TABLE III
Drugs for Prophylaxis
Drug
|
Dosage (mg/kg/ day)*
|
Remarks
|
|
Co-trimoxazole |
1-2 trimethoprim
50 sulphamethoxazole |
Maintain adequate fluid intake; avoid in infants under 6 weeks; contraindicated in G6PD
deficiency |
|
Nitrofurantoin (NFT) |
1-2 |
Considerable GI upset; contraindicated
in G6PD deficiency, infants <3 months, and in renal
insufficiency; resistance rare |
|
Cephlexin |
20 |
Use in young infants where administration of NFT and co-trimoxazole is restricted |
|
Methenamine mandelate |
75 in 2 divided doses |
GI upset, rash; not given with co-trimoxazole or in renal impairment |
* Administered as a single bedtime dose unless specified.
Compliance with chemoprophylaxis should be ensured on follow-up visits. The patient and the family need to be motivated and understand why they need to take the drug every day. Breakthrough UTI
may occur during prophylaxis, chiefly due to poor compliance and
occasionally secondary to infection by resistant organisms.
Prophylaxis is continued till the reflux resolves (see
Monitoring of course below). However development of new scars,
in kidneys previously showing normal growth, after 5 to 6 years
of age is unusual(23). Some experts propose that suppressive antibacterial therapy may be stopped in these children even if reflux persists(2,8,23-25)o If the child again returns with a febrile UTI, prophylaxis is resumed or the reflux surgically repaired.
Chemoprophylaxis should however be continued in patients with renal scars or frequently recurring UTI till resolution of VUR.
Surgical Treatment
The role of surgery in the treatment of VUR has been hotly debated since the 1960's. Surgical treatment of VUR consists of ureteric reimplantation.
Success rates exceeding 95% can be expected by those with
experience performing the various procedures(2,25). The chief complication of surgery is ureteral obstruction due to devascularization, kinking or torsion of the distal ureter which may occur in upto 4% patients; reflux may persist in 2.5%(26).
Results from prospective controlled studies comparing antibiotic prophylaxis and surgical repair for VUR are now available. They do not show any difference in the incidence of recurrent UTI,
progression of existing scars, or appearance of new scars in
surgically or medically treated patients. Renal growth was influenced not by the kind of therapy, but by the severity of renal scarring at initial assessment(4,27). Antireflux surgery did not also result in reduced incidence of hypertension or improvement of statural growth (10,21,25,28,29). Surgery is currently, therefore,
regarded as effective as medical management with no clear demonstration of benefits of one over the other.
Endoscopic treatment of reflux has gained acceptance in some countries(30). Polyteflon paste is injected in the sub- mucosa at the ureteral
orifice, thus bolstering it and curing the VUR. Other materials used include glutaraldehyde cross-linked collagen, silicone and alcohol. The results of endoscopic
correction are not as successful as traditional surgical techniques. Success rates of 60-80% are reported; recurrences of VUR may occur in 10% patients(31). The results are unsatisfactory with higher grades of reflux, duplication of the collecting system or neuropathic bladders. Concerns about the efficacy and long-term safety of synthetic implants has limited the acceptance of this procedure.
Choice of Therapy
All patients with VUR are initially placed on antibiotic prophylaxis with close monitoring for recurrences of UTI (see
below). This treatment usually suffices for most patients with mild (grades I-III reflux). Multiple recurrences of UTI while the child is receiving suppressive antibacterial therapy (i.e., breakthrough infections) are an indication for surgical repair. Surgical correction of VUR is also indicated in patients with: (i) grade IV or V reflux especially in children older than 2 years, since it is unlikely to resolve in these patients; (ii) non-compliance with medical management or intolerance to medication; (iii)
appearance of new renal scars or deterioration of renal function during medical therapy. Absence of compliance with medication may be a problem in our country and dictate consideration of surgery.
Surgery is also required if VUR is secondary to ureteral duplication or paraureteral diverticuli. Children with posterior urethral valves are managed initially by re- section of their valves; if reflux persists its definitive repair may also be needed. Patients with antenatally detected VUR
need to be followed closely. Therapy in these patients must be
individualized, taking into account the motivation of parents to administer continuous prophylaxis, need for repeated radiologic examinations and the presence or absence of renal scarring. Grade V or bilateral grade IV reflux in these children is unlikely to resolve and surgical repair is often necessary.
The management of persisting moderate to severe reflux in adolescent girls is
controversial. In view of its implications during pregnancy, persistent VUR in older girls may be repaired surgically. However, correction of VUR
does not guarantee prevention of bacteriuria or pyelonephritis in pregnancy(32). Therefore some experts recommend
surgical repair only in those adolescents showing clinical or scintigraphic evidence of pyelonephritis or development of new scars on follow-up(2).
Monitoring of Course
Follow-up urine cultures in asymptomatic patients, are apparently not necessary. Treatment of asymptomatic bacteriuria (ABU), even in patients with VUR, is not required and probably harmful(4). ABU is considered to be protective against infection with more pathogenic infections. Treatment of these patients does not improve renal growth or function, and may increase the risk of pyelonephritis,
perhaps by altering the balance of non-virulent and virulent micro-organisms.
Urine cultures should, however, be promptly obtained in presence of symptoms. Symptomatic UTI in children with VUR must be treated aggressively. Blood pressure, height, weight, and serum creatinine level is determined every 6 months. Voiding cystography (DRCG or MCU) is performed every 12-18 months. Antibiotic prophylaxis can be discontinued after reflux resolves. 'Some authors recommend that prophylaxis be continued till documentation of two negative cystograms performed a year apart. Renal US is done every year to monitor renal growth and cortical scarring. Follow-up DMSA scans are limited to children having break-through UTI. Appearance of new scars either on US or renal scan may suggest the need for surgical treatment.
General Measures
General measures for prevention of UTI are important. These include a liberal fluid intake, regular and complete bladder emptying, and local toilet. Constipation should be avoided. The wiping action in girls should be in an anterior to posterior direction to avoid fecal soiling of periurethral area. The child may be taught to void again after the end of micturition, so as to expel the refluxed urine entering the bladder.
|
Key Messages |
|
.
Important risk factors for pyelonephritis and reflux nephropathy are: VUR,
occurrence of UTI below 1 year of age, delay in treatment of UTI, and urinary tract obstruction.
.
All children with a UTI must be promptly evaluated for VUR or an underlying anatomic obstruction.
.
Mild to moderate reflux usually resolves with age.
. Antibiotic prophylaxis to maintain sterile urine while awaiting spontaneous resolution
of reflux is the cornerstone of treatment.
.
Surgical repair of VUR is as effective as antibiotic prophylaxis. Renal growth is influenced not by the kind of therapy, but the severity of renal scarring at initial assessement.
.
Surgical repair of VUR is preferred in: (i) patients with recurrent UTI despite prophylaxis; (ii)
those showing non-compliance to treatment; (iii) young children with grade IV or V reflux; and (iv) patients with VUR secondary to other urinary tract anomalies.
|
Management of Reflux Nephropathy
Patients with VUR who show hypertension or reduced renal function need careful evaluation. The presence of proteinuria especially when associated with renal scarring indicates progressive RN. Hypertension must be controlled and UTI treated. A restricted dietary intake of protein (1-1.5 g/kg per day; 60% high biologic value) is advised. Restriction of protein intake may retard progressive renal damage in these patients.
Treatment with angiotensin converting enzyme inhibitors (captopril, enalapril) is
associated with reduction of proteinuria and preservation of renal function(33). Pa- tients with RN showing features of chronic renal failure should be managed. using standard guidelines(34). The role of surgical repair of VUR in patients with RN and chronic renal failure is limited.
|
1.
Bollgren I, Winberg J. The periurethral flora in girls highly susceptible to urinary infections. Acta Pediatr Scand 1976; 65: 81-87.
2.
Belman AB. Vesicoureteric reflux. Pediatr Clin N Am 1997; 44: 1171-1190.
3.
Arant BS Jr. Vesicoureteric reflux and renal injury. Am
J
Kidney Dis 1991; 17: 491-
511.
4.
Linshaw M. Asymptomatic bacteriuria and vesicoureteral reflux in children. Kidney Int 1996; 50: 312-329.
5.
Steele BT, Demaria J. A new perspective on the natural history of vesicoureteric reflux. Pediatr1992; 90: 30-32.
6.
Arnold EP. Lower urinary tract abnormalities associated with vesicoureteric reflux. In: Second CJ Hodson Symposium on Reflux Nephropathy. Ed. Bailey RR. Sandoz, New Zealand, 1991; pp 45-48.
7.
Peters PC, Johnson DE, Jackson JH. Incidence of vesicoureteric reflux in the premature child.
J
Uro11967; 72: 259-260.
8.
White RH. Management of urinary tract infections. Arch Dis Child 1987; 62: 421- 427.
9.
Martinell J, Claesson, Lidin-Janson G, Jodal U. Urinary infection, reflux and renal scarring in females continuously for 13-38 years. Pediatr Nephro11995; 8: 131- 136.
10.
Velosa JA. Vesicoureteral reflux and reflux nephropathy. In: Primer on Kidney Diseases, 2nd edn.
Eds. Greenberg A, Cheung AK, Coffman TM, Falk RJ, National Kidney Foundation. San Diego, Academic Press, 1998;
pp 356-360.
11.
Martinell J, Jodal U, Lindin-Janson G. Pregnancies in women with and without renal scarring after urinary tract infection in childhood. Br Med
J
1990; 300: 840- 843.
12.
Torres VE, Velosa JA, Holley KE, Kellalis PP, Stickler GB, Kurtz SB. The progression of vesicoureteral reflux nephropathy. Ann Intern Med 1980; 92: 776-784.
13.
Jacobson SH, Eklof O, Eriksson CE, Lins L-E, Tidgren B, Winberg J. Development of hypertension and uremia after pyelonephritis in childhood: 27 years follow-up. Br Med
J
1989; 299:
703-706.
14.
Conway JJ, King LR. Belman AB, Thorson T Jr. Detection of vescioureteral reflux with radionuclide: A comparison study with roentegenographic cystography. Am
J
RoentgenoI1972; 115: 7-20.
15.
Rushton HG. The evaluation of acute pyelonephritis and renal scarring with technetium 99m-dimercaptosusccinic acid renal scintigraphy: evolving concepts and future directions. Pediatr Nephrol 1997; 11: 108-120.
16.
Gross GW, Lebowitz RL. Infection does not cause reflux. Am
J
Roentgenol 1981; 137: 929-932.
17.
Rosenberg AR, Kainer G, Srivastava T, Hughes C, Leighton O, Garrett W, et al. Long term followup of minimal collecting system dilatation detected in antenatal period. Pediatr Nephro11995; 9: C 26.
18.
Anderson P A, Rickwood AM. Features of primary vesicoureteric reflux detected by prenatal ultrasonography. Br
J
Uro11991; 67: 267-271.
19.
Chapman Cj, Bailey RR, Janus JD, Abbott GD, Lynn KL. Vesicoureteral reflux: Segregation analysis. Am
J
Med Genet 1985; 20: 577-584.
20.
Noe HN. The current status of screening for vesicoureteral reflux. Pediatr Nephrol 1995; 9: 638-641.
21.
Arant BS Jr. Medical management of mild and moderate vesicoureteral reflux:
Followup studies of infants and young children. A preliminary report of the
Southwest Pediatric Nephrology Group.
J
Urol 1992; 148: 1683-1687.
22.
Huang F-Y, Tsai T-C. Resolution of vesicoureteral reflux during medical management of children. Pediatr Nephrol 1995; 9: 715-717.
23.
Vernon SJ, Coulthard MG, Lambert HJ, Keir MJ, Mathews JN. New renal scarring in children who at age 3 and 4 years had normal scans with dimercaptosuccinic acid: Follow up study. Br Med
J
1997; 315: 905-908.
24.
Goldraich NP, Goldraich IH. Followup of conservatively treated children with high and low grade vesicoureteral reflux study.
J
Urol 1992; 148: 1688-1692.
25. Birmingham Reflux Study Group: Prospective trial of operative versus non operative treatment of severe vesicoureteric reflux in children: Five years observation. Br Med
J
Clin Res Edu 1987; 295: 237- 241.
26.
Hjalmas K, Lohr G, Tamminen-Mobius T, Seppanen J, Olbing H, Wikstrom S. Surgical results in the International Reflux Study in Children (Europe). J Urol 1992; 148: 1657-1661.
27.
Aggarwal VK, Verrier Jones K, Asscher A W, Evans C, Williams LA. Covert bacteriuria: Long term followup. Arch Dis Child 1991; 66: 1284-1288.
28.
Wallace OM, Rothwell DL, Williams 01. The long term follow up of surgically treated vesicoureteral reflux. Br
J
Urol 1978; 50: 479-484.
29.
Smellie JM, Tamminen-Mobius T, Olbing H, Classon I, Wikstad I, Jodal U, et al.
Five year study of medical or surgical treatment in children with
severe reflux: Radiologic renal findings. The International Reflux Study in Children. Pediatr
Nephro11992;
6: 223-230.
30.
O'Donnell B, Puri P. Endoscopic correction of primary vesicoureteric reflux: results in 94 ureters. Br Med
J
1986; 293: 1404-1406.
31.
Ritchey ML, Bloom D. Report of the American Academy of Pediatrics Section of Urology Meeting. Pediatr Nephrol 1995; 9: 642-646.
32.
Mansfield JT, Snow BW, Cartwright PC, Wadsworth K. Complications of pregnancy in women after childhood reimplantation for vesicoureteral reflux:
An update with 25 years follow up.
J
Urol 1995;154:787-791.
33.
Lama G, Salsano ME, Pedulla M, Grassia C, Ruocco G. Angiotensin converting enzyme inhibitors and reflux nephropathy: 2 years followup. Pediatr Nephrol 1997; 11: 714-718.
34.
Srivastava RN, Bagga A. Chronic renal failure In: Pediatr Nephrology, 2nd end. Eds. Srivastava RN, Bagga A. New Delhi; 1997; pp 164-174.
|
