|
M.N. Muranjan,
B.A. Bharucha,
M.V. Kirtane,
C.T. Deshmukh
From the Departments of Pediatrics and Otorhino- laryngology*, Seth C.S. Medical College and K.E.M. Hospital, Mumbai, India.
Reprint requests.~ Dr. M.N. Muranjan, Lecturer of Pediatrics K.E.M. Hospital, Parel,
Mumbai 400012, India.
Manuscript Received: July 7, ]998; Initial review completed: August 6. 1998;
Revision Accepted: September 26, 1998.
Meningitis
associated with cerebrospinal fluid (CSF) leaks is a common sequel of
traumatic fractures or surgery resulting in CSF otorrhea or rhinorrhea. In
absence of a precipitating event, CSF leaks can' occur
spontaneously through congenital fistulae in the region of the labyrinth and
base of the anterior cranial fossa (1), especially when associated with
congenital anomalies(2). The Mondini dysplasia is one such rare anomaly,
which usually remains undiagnosed until deafness or recurrent meningitis
warrants radiological imaging. We report one such child in whom bilateral
Mondini dysplasia was detected during the course of investigations for
recurrent meningitis.
Case Report
The patient was a 4-year-old male, offspring of a nonconsanguineous
marriage, delivered at term after an uncomplicated pregnancy.
He was born centrally cyanosed, with two loops of the umbilical cord around the
neck and signs of mild asphyxia within the first 24 hours. The subsequent
postnatal period was unremarkable, however there was a lag in mental as well as
motor development. Social smile was attained by 6 months. The child started
reaching out for objects at 6 months. By 12 months head control had been
achieved, whereas sitting unaided and independent walking were attained by 15
and 18 months, respectively. The child was making vowel sounds by 18 months,
could point to familiar objects by 3 years, but even at 4 years was unable to
speak in sentences.
The first documented episode of meningococcal meningitis was at 3 years of age;
followed by a second, 6 months later. On both the occasions he was adequately
treated at a local hospital with 14 days of intravenous Ceftriaxone. He had
received 2 doses of meningococcal vaccine and oral Rifampicin prophylaxis, which
failed to prevent a third recurrence and prompted a referral for
investigations. During this admission, on careful probing the parents denied
history of antecedent trauma or recurrent infections. The child was noted to be
microcephalic, but other anthropometric parameters were normal. There were no
facial dysmorphisms, physical or systemic abnormalities. Neurologically, tone,
power, deep tendon reflexes and plantar responses were normal. Except for a left
LMN type of facial paresis, other cranial nerve examination was normal.
He was tested for immunolgic abnormalities. Total white cell count was 11000/cumm,
absolute lymphocyte count was 3520/cumm with T cells constituting 65% of
the population. Leucocyte phagocytosis opsonisation and nitroblue tetrazolium
reduction test were normal (8 f%, 61 % and 100%, respectively). The levels of
IgG, IgM and IgA were within the normal range for age and serum C3 and C4 levels
were normal. ELISA test for HIV was nonreactive. Having ruled out
immunologic abnormalities, he was. referred for an Otolaryngologic evaluation
which revealed bilateral profound sensorineural hearing loss (SNHL) on BAER
testing. Bilatenil Mondini malformation of the cochlea (more severe 'on the
right) was diagnosed on temporal bone high resolution CT (HRCT) scans (Fig.
1) with otitis media and mastoiditis of the' left. ear. A cisternography with
metrizamide' detected CSF fistula through the left internal aCQustic canal (lAC).
A left ear exploration was done. No defect or leak could be demonstrated at the
time of surgery through tegmen plate, sinus plate, round window or oval window.
The ossicular chain was intact but the stapes was thick; almost a solid piece of
bone, and fixed. The round window reflex was absent, confirming fixation of the
stapes. Since no obvious leak was apparent, the probable sites of leak were
sealed off. The child was discharged a week later.
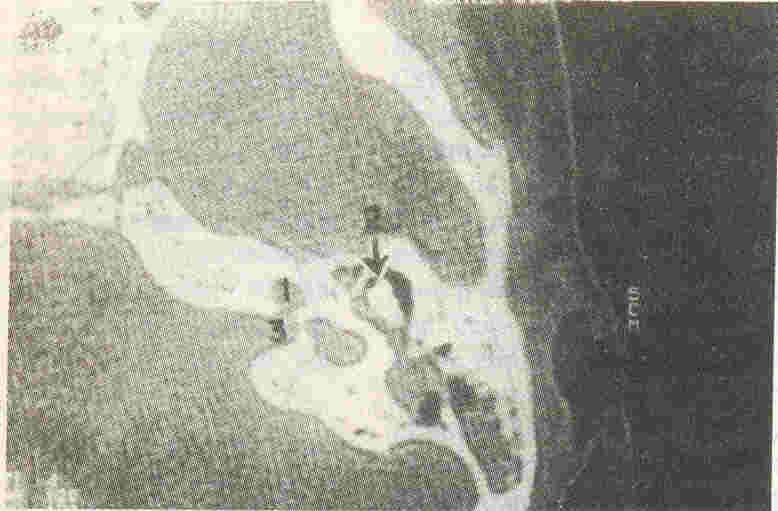 |
|
Fig.1. HRCT
showing the mondini dysplasia. The cochlea (1) is represented by a
sac-like remnant; (2) = middle ear cleft with "ice-cream" cone
appearance of ossicles; and (3) = internal acoustic meatus |
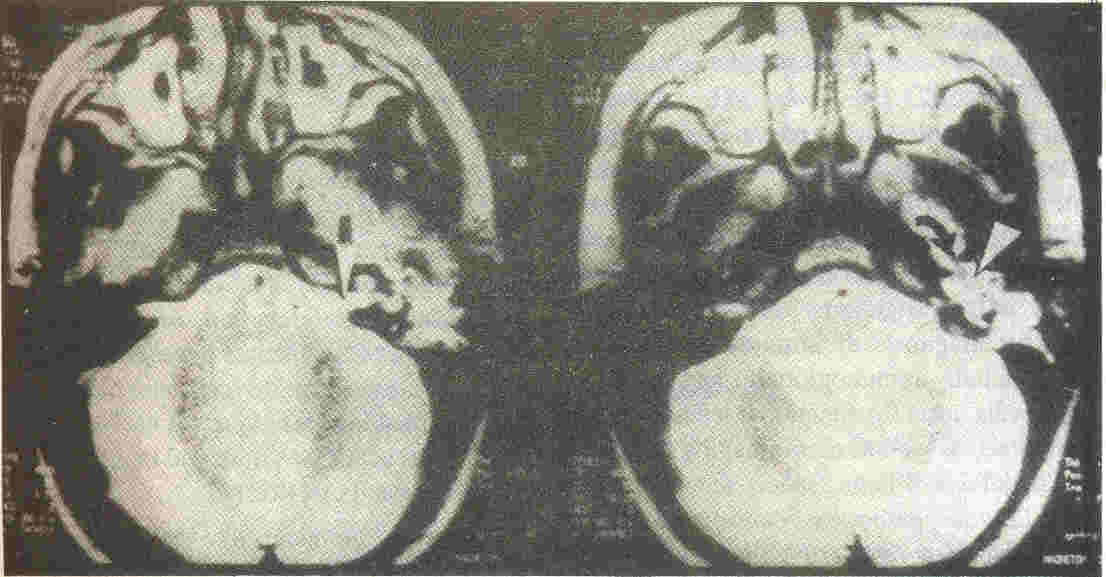 |
|
Fig. 2. CSF leak
along the left vestibulocochlear nerve through a widely patent IAC
(while on MRI T2 weighted image), indicated by arrow.
On the right side, SCF is normally seen stopping short at the
fundus of the vestibulocochlear nerve. CSF is visualized tracking
through the eustachian tube (curved arrow) and filling up the
middle ear cleft (arrow head) |
Unfortunately, meningitis recurred at 4 years of age. This time a MRI was
advised. The MRI now precisely defined the site of leak on the left
through a patent lAC via a deficient fundus into a dysplastic vestibule and CSF
was seen filling the. middle ear cleft and. tracking down the left eustachian
tube (Figs. 2 & 3). The child was subjected to re-exploration of the left
ear. The leak was apparent through the stapes footplate. The facial nerve in
this area (second genu) was dehiscent and abutting against the stapes. Since
there was total deafness on the left, the stapes along with the footplate was
removed and CSF was seen gushing through the oval window. The leak was sealed
appropriately. Post operatively, the child developed facial paresis on the left.
After a 4 week course of intravenous Ceftriaxone with steroids and a documented
CSF cure, he was discharged. He was seen 6 months following surgery. The
facial paresis had not recovered completely, but there was no recurrence of
meningitis; though the period was too short to be of significance.
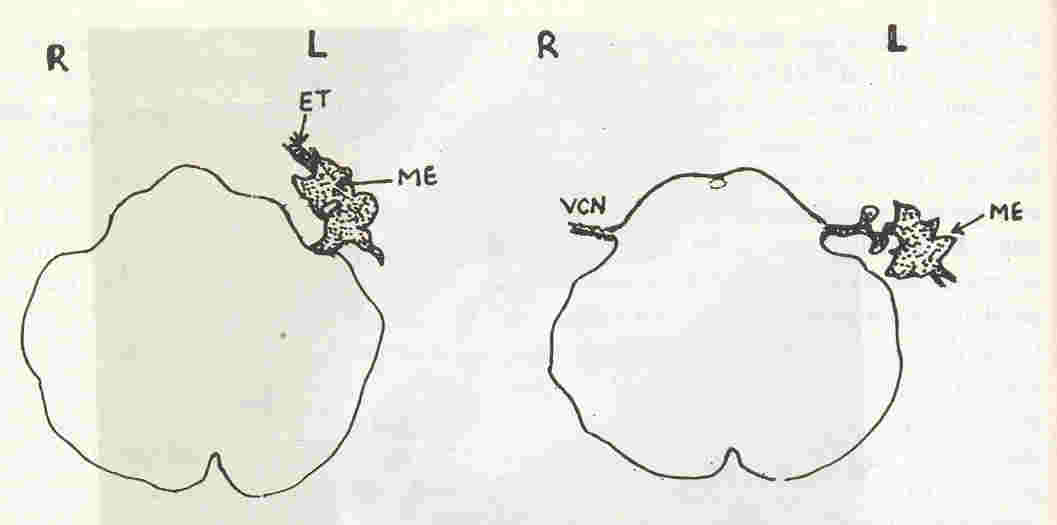 |
|
Fig. 3.
Simplified
sketch
of the MR image. The CSF (depicted as a stippled area) along the
leftvestibulocochlear nerve
(VCN) leaks into the middle ear cleft (ME) and along the eustachian tube
(ET). On the right, the subarachnoid space with the CSF surrounding the VCN is
ending normally at the fundus of the nerve. L
=
Left, R
=
Right. |
Discussion
In the antibiotic era, meningitis is rarely difficult to treat or eradicate; but
when recur- rent, it poses a diagnostic challenge. In absence of a breach in
mucocutaneous barriers serving as portals for infecting organisms, a battery of
investigations need to be under- taken for diagnosis of immune deficiencies
which include immunoglobulin and complement levels, tests for functional
leucocyte abnormalities, as well as neuroimaging to detect cranial defects. .Meningococci
and pneumococci are the pathogens causing recurrent meningitis as a result of
complement deficiency, functional or anatomic asplenia, hypogammaglobulinemia
and cranial defects(3).
Our child presented with recurrent meningococcal meningitis. Investigations
excluded immunologic incompetence. The inner ear anomaly, associated
CSF -
perilymph fistula via the lAC and CSF leak into the middle ear and
eustachian tube was demonstrated by modem neuroimaging, namely HRCT and MRI.
Cranial defects and the resultant CSF fistulae are notoriously difficult to
detect radio- logically(1,4). Traditional modalities for diagnosis of inner ear
anomalies such as skull polytomography(5) and intrathecal instillation of
fluorescent dyes and radioisotope tracers to detect CSF fistulae have given way
to CT with metrizamide cistemography and MRI as imaging techniques of
choice(4,6-8). Anomalies of the labyrinth and middle ear are best diagnosed on
HRCT(2).
Inner ear anomalies like the Mondini dysplasia with CSF fistula should be
suspected in deaf children with recurrent meningitis(4,7), especially those
having stigmata of syndromes such as Klippel-Feil, Pendred, DiGeorge and
chromosomal trisomies(7,9). In our patient, the malformation occurred in
isolation, there being no characteristic features of any of the preceding
syndromes.
The classical Mondini malformation consists of 11/2 cochlear
coils instead of the usual 21/2, a cystic
dilatation of the common apical chamber with absence of
interscalar septum between the middle and apical coil and a hypoplastic modiolus.
Etiologically it is believed to result from a developmental arrest at the sixth
or seventh week of embryonic life possibly due to
teratogenicinsults(9,10). The anatomic and histopathologic characteristics have
been reviewed extensively in otolaryngologic literature(5,7).
A variety of otologic anomalies have been described; involving
semicircular canals, vestibule, endolymphatic sac and duct, ossicular chain,
facial canal, round window, oval window and IAC(8,l0). Our patient was
noted to have a dehiscent facial canal at surgery. The stapes was
abnormal, with a thick- ened suprastructure. A defect in the footplate served as
a site for pathogens to gain access to the subarachnoid space. The lAC was
widely patent, resulting in CSF-perilymph fistula along the perineural sheath
of the vestibulocochlear nerve. Similar defects of the stapes and lAC
have been described in ears with
the Mondini malformation presenting with recurrent meningitis(2,4,6,7).
These children may be referred for hearing loss (HL), but often it is to the
pediatrician that they present with recurrent meningitis. There is a record
of 29 admissions with 20 attacks of meninigitis in a child before the
underlying anomaly was suspected and diagnosed(4). The HL is sensorineural,
resulting from abnormalities of organ of Corti, and progressive
due to altered pressure in the perilymphatic space as a result of
its communication with the subarachnoid space(2). Mixed HL may also occur, the
conductive component either due to ossicular abnormality or CSF otorrhea. In
fact, conductive deafness due to fluid in the middle ear is often not perceived,
occurring as it does in a ear deaf due to Mondini dysplasia. In presence of
an intact tympanic membrane, CSF in middle ear does not manifest as otorrhea;
but may be seen trickling through the eustachian tube orifice on
nasopharyngoscopy or manifesting as rhinorrhea if the leak is profuse(7). The
child's parents had not noted the deafness, possibly attributing the speech
delay as part of overall developmental lag. Both on otoscopy and
nasopharyngoscopy, no CSF leak could be detected. Though mental retardation has
not been reported with isolated Mondini malformations, its presence in our
patient could probably be a sequel of the hypoxic-ischaemic event
suffered during labor.
Though rare, inner ear anomalies must be suspected when recurrent meningitis
occurs in a deaf child (particularly when deafness is unilateral) and in
children with certain craniofacial syndromes known to be associated with SNHL;
especially in the absence of antecedent trauma. The associated
CSF-perilymph fistula and leak are best diagnosed on MRI ot CT with metrizamide
cisternography. Surgery may not always result in definitive cure, multiple
attempts often being necessary(4,1l). Whenever feasible amplification aids must
be prescribed since some residual hearing may be preserved(10).
Acknowledgement
The authors wish to thank Dr. P.M. Pai, Dean of the Seth G.S. Medical
College & K.E.M. Hospital, Mumbai, for granting per- mission to publish this
article.
|
1. Gacek RR, Leipzig B. Congenital
cerebrospinal otorrhea. Ann Otol Rhinol Larygnol 1979; 88:
358-365.
2. Curtin HD. Congenital malformations of the ear. Otolaryngol
Clin North Am 1988; 21: 217- 336.
3. Prober CG. Infections of the Central Nervous System. In:
Nelson Textbook of Pediatrics, 15th edn. Eds. Behrman RE,
Kliegman RM, Arvin AM. Bangalore, Prism India Ltd, 1995; pp
707-713.
4. Stool S, Leeds NE, Shulman K. The syndrome of congenital
deafness and otic meningitis: Diagnosis and managment. J
Pediatr 1967; 71: 547-552.
5. Paparella MM. Mondini's deafness: A review of
histopathology. Ann Otol Rhinol Laryngol 1980; 89 (SuppI67):
1-10.
6. Biggers WP, Neil Howell N, Fischer NO, Himadi G, Hill C.
Congenital ear anomalies associated with otic meningitis. Arch
Otolaryngol1973; 97: 339-401.
7. Schuknecht HF. Mondini dysplasia. A clinical and
pathological study. Ann Otol Rhinol Laryngol 1980; 89 (Suppl
65): 1-23.
8. McGuirt WF (Jr.), Stool SE. Cerebrospinal fluid fistula:
The identification and management in Pediatric temporal bone
fractures. Laryngoscope 1995; 105: 361-362.
9. Chan KH. Sensorineural hearing loss in children,
Classification and evaluation. Otolaryngol Clin North Am 1994;
27: 475.
10. Paparella MM, Fox RY, Schachem PA. Diagnosis and Treatment
of sensorineural loss in children. Otolaryngol Clin North Am
1989; 22: 63-64.
11. Farrior JB, Endicott IN. Congenital mixed deafness:
Cerebrospinal fluid otorrhea. Ablation of the aqueduct of the
cochlea. Laryngoscope 1971; 81: 694.
|
