|
|
|
Indian Pediatr Suppl 2009;46: S37-S42 |
 |
Pyritinol for Post-asphyxial Encephalopathy in
Term Babies – A Randomized Double-Blind Controlled Trial |
|
MKC Nair, Babu George and L Jeyaseelan*
From Child Development Centre, Medical College,
Thiruvananthapuram, Kerala, India and
*Department of Biostatistics, Christian Medical College, Vellore,
Tamilnadu, India.
Correspondence to: Dr MKC Nair, Professor of Pediatrics
and Clinical Epidemiology and,
Director, Child Development Centre, Medical College, Thiruvananthapuram
695 011, Kerala, India.
E-mail: [email protected]
|
Abstract
Objective: To evaluate the efficacy of
pyritinol in improving the neurodevelopmental outcome at one year of age
among term babies with post-asphyxial encephalopathy.
Setting: Level II Neonatal Nursery and Child
Development Centre, Medical College, Thiruvananthapuram.
Design: Randomised placebo controlled double
blind trial.
Participants: 108 term babies with post-asphyxial
encephalopathy, stratified into three grades based on clinical criteria.
Intervention: The treatment group (n=54) received
pyritinol and the control group (n=54) received placebo, in exactly the
same increasing dosage schedule of 1 to 5mL liquid drug (20-100 mg) from
8th postnatal day until the end of six months.
Outcome variables: Mean Mental Development Index
(MDI) and mean Psychomotor Development Index (PDI) measured on Bayley
Scales of Infant Development at one year of age.
Results: No statistically significant difference
was observed in MDI or PDI scores at one year between the treatment and
control groups. The confidence interval for the differences ranged from
–6.3 to+8.7 for MDI and from –4.1 to+12.7 for PDI. On multiple
regression analysis using one year MDI and PDI scores, even after
controlling for birthweight, there was no statistically significant
difference between the treatment and control groups.
Conclusion: Pyritinol is not useful in improving
the neurodevelopmental status of babies with post-asphyxial
encephalopathy at one year of age.
Keywords: Neurodevelopmental outcome, Post- asphyxial
encephalopathy, Pyritinol.
|
|
P
erinatal hypoxia has been
recognized as a possible cause of mental and physical handicaps in
childhood for more than 100 years(1). Approximately 23% of the 4 million
annual global neonatal deaths are attributable to birth asphyxia(2). In a
prospective cohort study undertaken in the principal maternity hospital of
Kathmandu, an upper estimate for the prevalence of major neuro-impairment
at 1 year, attributable to birth asphyxia was 1 per 1000 live births(3).
The best predictive risk factors for the neurological prognosis at
follow-up is reported to be severe perinatal asphyxia at birth and/or
evidence of encephalopathy in neonatal period(4,5). Newborn encephalopathy
(Grades I, II and III), particularly with seizures and recurrent apnea,
has been demonstrated to be an important predictor of subsequent motor and
cognitive handicaps(6-9). Clinical presentation of birth asphyxia with
severe newborn depression has demonstrated that most children who survived
with sequelae had clinical signs of encephalopathy during the neonatal
period(10).
Pyritinol or pyritinol dihydrochloride mono-hydrate, is
a derivative of pyridoxine with a chemical structure of two pyridoxine
molecules linked by a disulphide bridge and has only 0.1% of vitamin B6
activities. Benesova, et al.(11), based on a controlled trial of
pyritinol, reported significant improvement in neurodevelopmental outcome
at one year and every year after that till six years of age. However, this
was not a controlled trial and hence the results, not conclusive. The drug
was effective only if used early and for prolonged periods(12). This drug
is not routinely used in our hospital nursery and during follow up,
although pediatricians may use it in individual cases. Only a randomized
controlled double blind trial can give a definite answer on the efficacy
of pyritinol in perinatal asphyxia.
The broad objective of the study was to evaluate the
efficacy of pyritinol in improving the neurodevelopmental outcome at one
year of age among term babies with post-asphyxial encephalopathy, using
Bayley Scales of Infant Development (BSID), the most widely accepted
objective developmental assessment tool(13). The hypothesis tested in this
randomized placebo controlled double blind trial was that the mean Mental
Developmental Index (MDI) and the mean Psychomotor Developmental Index
(PDI) scores obtained in BSID, for the group of term post-asphyxial
encephalopathy babies receiving pyritinol is greater than for the control
group of babies.
Methods
A sample size of 54 in each group was calculated to
detect a mean clinically significant difference of 16 in the MDI or PDI
scores for two-tailed test with 90% power, significance level at 0.05 and
with 5% drop-out rate(14). A pilot study was done on a sample of 15 babies
with post-asphyxial encephalopathy.
The sequential criteria for inclusion in the study
were: born in the labor room of Medical College, Thiruvananthapuram;
completed 37 weeks of gestation; admitted to the special care nursery with
a clinical diagnosis of birth asphyxia; clinical evidence of
encephalopathy observed in the first 7 days of postnatal life; alive on
8th postnatal day; and, parents agreeing to randomization and monthly
follow-up. The specific exclusion criteria were, total serum bilirubin
more than 15mg/dL on the 7th postnatal day, blood glucose level less than
30mg/dL recorded on two occasions 4 hours apart any time during the first
7 days of postnatal life, neonatal meningitis in the first 7 days of life,
chromosomal anomaly, microcephaly, hydrocephalus, and any congenital
anomaly known to affect growth and development.
The study was conducted with the approval of the
ethical committee of the Medical College, Thiruvananthapuram. After
obtaining the informed consent, participants were randomized on the 8th
post natal day into treatment and control groups, using block
randomization and separate random number series for the three grades to
get the same number of treatment and control patients in each grade (I, II
and III) of post-asphyxial encephalopathy. Serially numbered opaque
envelops, containing the allocation detail of a subject were developed at
the Department of Biostatistics, Christian Medical College, Vellore.
Both the groups of babies received similar treatment as
per the routine practice of the hospital till the 8 th
postnatal day. Eligible cases received, according to the randomized
coding, placebo or active drug (pysitimol, 1 mL=20 mg) both having same
consistency, colour and smell and the only difference being the batch
number. The same dosage schedule was followed for drug and placebo and
given orally as a single dose in the morning so as to avoid any possible
sleep disturbances. A calibrated 1 ml medicine dropper was supplied for
ease of administration of correct dose at home.
The increasing dosage schedule included 1 mL of the
solution (placebo/pyritinol) per day from day 8 to day 30, increased by 1
mL every 15 days to 5 mL per day by day 76. This amount of the solution
was continued till six months of age.
The parents were given a detailed discharge summary and
a special Child Development Centre monthly follow-up schedule card.
Contamination was avoided by checking the details of any medication
received for more than one week. The medication bottle was brought along,
collected and measured as an indicator of compliance. During the monthly
follow-up visit, the mother was asked about any problem the baby had in
the previous month and a thorough physical examination was done,
specifically looking for any possible side effect of the drug. Any rash
resembling a drug rash, clinical jaundice after one month of age, clinical
increase in liver size at least 2cms more than on the previous visit and
proteinuria (qualitatively reported as 2+ or above) were specifically
noted.
The one year primary outcome measurement was Mental
Development Index and Psychomotor Development Index obtained on the Bayley
Scales of Infant Development (BSID), Baroda, India norms. BSID was
administered in a separate quiet room by a well-trained observer blind to
the treatment status of the babies. Every effort was made to adhere to the
detailed instructions of the BSID test manual(13). The test items were
presented in order of difficulty and those items passed were ticked, but
added up only at a later stage to avoid any test bias. The raw scores
obtained separately for mental scale and motor scale, by adding together
the number of items passed on each scale separately, were then converted
to MDI and PDI scores, respectively, using conversion tables provided in
the test manual. Secondary outcome measurements at one year included
weight taken without clothes, using a beam type of weighing machine
calibrated against a standard weight once-a-week, length taken using a
simple infantometer, (with the baby supine, knees together and legs in a
straight position) and, head circumference measured using a metal tape
running through the most prominent part posteriorly and just above
glabella anteriorly.
The criteria for loss to follow-up was not reporting
for 1 year assessment of outcome variables even after sending two
reminders to the home address and one to the alternative address at 7 days
interval and evidence of having left the place. Quality check of the data
being collected was done by perusal of individual data sheets at the
weekly meetings of the research team. Out of 108 babies randomised on the
8 th postnatal day, 100 babies
(treatment group 51, control group 49) with outcome measurements available
at one year, were included in the initial "intention to treat" analysis.
All 100 babies had received the trial medication for periods of 75% or
more of the total days the baby should have received the medication.
Therefore, there was no need to consider any separate analysis of those
with completed treatment. An analysis of the baseline characteristics was
done using chi-square statistic and student’s t-test to look for
any statistically significant differences between the treatment and
control groups. To test the hypothesis regarding MDI and PDI, Student’s
t-test was used to compare means of the two independent samples, after
seeing that the data were approximately normally distributed. A 95%
confidence interval for the true difference in population means was also
calculated. Multiple regression analysis using MDI and PDI scores at one
year of age was done with pyritinol/placebo and any baseline variable with
significant difference between the groups, as explanatory variables.
The double blind nature of the study was strictly
maintained at all stages of the trial. An independent person kept the
coding about the treatment status of the patient. The parents of the
babies, the neonatal consultant, the investigator, the research assistants
and the outcome evaluators did not know the treatment status of the
babies.
Results
During the recruitment period of 17 months there were a
total of 21,604 deliveries, with 349 babies with diagnosis of asphyxia. Of
these, 155 did not develop post-asphyxial encephalopathy, 20 babies
meeting exclusion criteria were excluded and 62 babies died in the labor
room or nursery, leaving 112 cases of post-asphyxial encephalopathy
available for randomi-sation on the 8th postnatal day. Excluding four
babies whose parents expressed inability to come for monthly visits, 108
babies were randomised (Fig. 1). After block randomisation
we could obtain only 5 each of grade II and 2 each of grade III
encephalopathy, possibly because it is babies with severe asphyxia who are
likely to develop grade II and III encephalopathy, who die early and
cannot be saved without ventilator support.
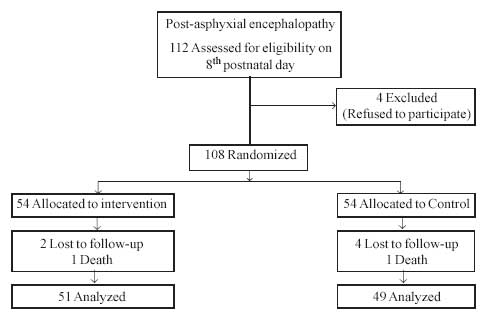 |
|
Fig. 1 Flow diagram of patients in the
study. |
Table I shows comparison of baseline
characteristics of the two groups at the time of randomization. There was
a statistically significant difference observed for the weight at birth
between treatment and control groups. Table II shows no
statistically significant difference in MDI or PDI scores at one year
between the treatment and control groups. There were also no statistically
significant differences observed between the treatment and the control
groups on growth parameters of weight, height and head circumference. As a
statistically significant difference was observed for the weight at birth
between treatment and control groups, adjustment for this potential
confounder was needed in the analysis of outcomes. On multiple regression
analysis using one year MDI and PDI scores, even after controlling for
birth weight there was no statistically significant difference between the
treatment and control groups.
TABLE I
Comparison of Treatment and Control Groups at Randomization
| Characteristics |
Treatment (n=54) (SD) |
Control (n=54) (SD) |
P value |
| Encephalopathy |
|
|
1.0 |
| Grade I |
47 |
47 |
|
| Grade II |
5 |
5 |
|
| Grade III |
2 |
2 |
|
| Abnormal delivery |
27 |
27 |
1.0 |
| Male: female ratio |
32:22 |
34:20 |
0.34 |
| Low birthweight (<2500g) |
11 |
15 |
0.49 |
| Low SE status |
40 |
43 |
0.64 |
| Mean weight at birth |
2841 (513.1) |
2627 (461.3) |
0.02 |
| Mean length at birth |
48.1 (2.8) |
48.0 (1.8) |
0.76 |
| Mean head circumference at birth |
33.7 (1.9) |
33.2 (1.1) |
0.10 |
| Mean gestational age |
39.1 (1.3) |
39.1 (1.3) |
0.95 |
| Days of nursery stay (mean) |
3.7 (2.1) |
4.0 (3.6) |
0.70 |
| Mother’s education (years) (mean) |
7.5 (3.2) |
8.2 (2.9) |
0.31 |
| Father’s education
(years) (mean) |
7.4
(2.6) |
8.1
(2.6) |
0.18 |
TABLE II
Comparison of Mental Development Index Score, Psychomotor Development Index
Score and Growth Parameters at 1 Year
| Score |
Treatment Group
(n=51)
Mean (SE) |
Control Group
(n=49)
Mean (SE) |
Difference in Means
(95% CI)
|
P value |
| Mental Development Index |
91.6 (2.9) |
92.8 ( 2.5) |
1.2 (–6.3, +8.7) |
0.75 |
| Psychomotor Development Index |
96.0 (3.2) |
100.3 (2.7) |
4.3 (–4.1, +12.7) |
0.31 |
| Weight (kg) |
8.6 (0.2) |
8.6 (0.2) |
0.0 (–0.5, +0.5) |
0.86 |
| Length (cm) |
71.9 (1.4) |
72.1 (1.5) |
0.2 (–3.8, +0.5) |
0.94 |
| Head circumference (cm) |
44.8 (0.2) |
45.0 (0.2) |
0.2 (–0.4, +0.8) |
0.63 |
Discussion
As physicians we always feel inadequate if we cannot
offer drugs for a medical problem that we face and birth asphyxia is no
exception. The drug pyritinol is widely used among asphyxiated babies by
practitioners in India, South East Asia and some East European countries,
even without strong evidence and hence this study is timely and
appropriate. Availability of good objective outcome measurements is
crucial for successful completion of any good trial. The objective
neurodevelopmental measurement of MDI and PDI using Bayley scales of
infant development standardized for the Indian population were used as the
one-year outcomes in this study(14).
One of the main issues that we have to face when we try
to relate asphyxia and outcome is the problem of defining post-asphyxial
brain damage(15). We chose post- asphyxial encephalopathy as marker of
asphyxia in this study because of the dual advantage of fairly accurate
prediction of outcome and generalisability, as majority of neonatal units
in India are level II without ventilation facilities.
This trial has failed to show any evidence to suggest
that pyritinol is useful in improving the neurodevelopmental status at one
year of age. For any negative trial result it is important to consider the
following methodological issues. Firstly, did the study have adequate
power? The sample size calculated to detect a clinically significant
difference of 16 MDI or PDI score with 90% power at 0.05 significance
level was 96 and we have one year out come measurements available for 100
babies. Secondly, did the study get adequate drug compliance? For all the
babies for whom one-year outcome measurements were made, there was
evidence that they had consumed more than 75% of the total expected drug/
placebo. Thirdly, did the study have mortality and or complications? There
were two deaths one on the 58th day, a case of grade-III encephalopathy
belonging to the control group, and the other baby belonging to the
treatment group died at home due to bronchopneumonia, both likely to be
unrelated to use of drug. None of the babies belonging to either the
pyritinol or the control group had evidence of drug complications. This
was the same as observed in the Prague study(11), suggesting that the drug
is safe to be used in infants at doses below 100 mg per day.
Retrospective power calculations were done for MDI and
PDI. The current power is 6% and 19% respectively. At the planning stage,
the study group expected minimum of 16 units difference between the two
arms as this was clinically meaningful difference and we wanted to
achieve. However, the current power suggested that the Pyritinol arm is as
good as control arm. This implied fact that the effect due to Pyritinol is
absolutely nil. It is also evident that failing to reject the null
hypothesis is not due to lesser numbers. Moreover, it is unwise to show a
small difference in MDI to be statistically significant by recruiting
thousands of children.
This study has failed to show any positive effect of
pyritinol in improving the neurodevelopmental status of babies with
post-asphyxial encephalopathy at one year of age. Therefore, widespread
use of the drug should be discouraged not only for economic reasons but
also for ethical reasons.
Acknowledgments
Director and faculty of Centre for Clinical
Epidemiology and Biostatistics, University of New Castle, Australia; Staff
of Neonatal Nursery; Asokan N, and other staff of Child Development
Centre, Medical College, Thiruvananthapuram.
Contributors: MKCN was involved in designing the
study and preparation of the manuscript and will act as guarantor. BG was
involved in the data collection and manuscript writing, LJ was involved in
analysis of data.
Funding: M Med Sc Research Grant, University
of New Castle.
Competing interests: None stated. The findings and
conclusions in this study are those of the authors and do not necessarily
represent the views of the funding agency.
|
What the Study Adds?
• Pyritinol does not improve neurodevelopmental
status of babies with post-asphyxial encephalopathy at one year of
age. |
References
1. Little WJ. On the influence of abnormal parturition,
difficult labour, pre-mature birth and asphyxia neonatorum on the mental
and physical condition of the child; especially in relation to
deformities. Transact of the Obst Soc London 1862; 3: 293-344.
2. Lawn JE, Cousens S, Zupan J. 4 million neonatal
deaths: When? where? why? Lancet 2005; 365: 891-900.
3. Ellis M, Manandhar N, Shrestha PS, Shrestha L,
Manandhar DS, Anthony M. Outcome at 1 year of neonatal encephalopathy in
Kathmandu, Nepal. Dev Med Child Neurol 1999; 41: 689-695.
4. Gonzalez de Dios J, Moya M. Perinatal asphyxia,
hypoxic-ischemic encephalopathy and neuro-logical sequelae in full-term
newborns. II. Description and interrelation Rev Neurol 1996; 24: 969-976.
5. Gonzalez de Dios J, Moya M, Vioque J. Risk factors
predictive of neurological sequelae in term newborn infants with perinatal
asphyxia. Rev Neurol 2000; 32: 210-216.
6. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following foetal distress. Arch Neurol 1976; 33: 696-705.
7. Fenichel GM. Hypoxic ischemic encephalopathy in the
newborn. Arch Neurol 1983; 40: 261-266.
8. Nelson KB, Ellenberg JH. Neonatal signs as
predictors of cerebral palsy. Pediatrics 1979; 64: 225-232.
9. Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA,
Karchmar EJ. The predictive significance of biologic risk factors for
deficits in children of a high-risk population. Am J Obstet Gynecol 1983;
145:1059-1068.
10. Scott H. Outcome of severe birth asphyxia. Arch Dis
Child 1976; 51: 712-716.
11. Benesova O, Petova J, Vinsova N (Eds.). Perinatal
distress and brain development. Prague: Avicenum, Czechoslovak Medical
Press 1980. p. 52-130.
12. Petova J, Vinsova N, Benesova O. Psychomotor
development of high risk neonates during early and long-term
administration of pyritinol. Cesk Neurol Neurochir 1979; 42: 402-412.
13. Phatak P (Ed). Manual for using Bayley Scales of
Infant Development based on Baroda studies and Baroda norms, India: MS
University of Baroda 1973.
14. Dobson AJ. Calculating sample size. Transactions of
the Menzies Foundation 1984; 7: 75-79.
15. Shankaran S, Woldt E, Koepke T, Bedard MP, Nandayal R. Acute
neonatal morbidity and long-term central nervous system sequelae of
perinatal asphyxia in term infants. Early Hum Dev 1991; 25: 135-148. |
|
|
 |
|

