|
|
|
Indian Pediatr 2021;58: 820-825 |
 |
Efficacy of Daily Supplementation of Milk
Fortified With Vitamin D2 for Three Months in Healthy School
Children: A Randomized Placebo Controlled Trial
|
Raman Kumar Marwaha,1
Aashima Dabas,2
Seema Puri,3
Mani Kalaivani,5
Vineet Dabas,4
Sangeeta Yadav,2
Arjun Dang,6 R
Pullakhandam,7
Sushil Gupta,8
Archana Narang9
From 1Society for Endocrine Health Care of Elderly,
Adolescents and Children, New Delhi; Departments of
2Pediatrics and 4Orthopedics, Maulana Azad Medical College
and Lok Nayak Hospital, New Delhi; 3Food and Nutrition,
Institute of Home Economics, University of Delhi;
5Biostatistics, All India Institute of Medical Sciences, New
Delhi; 6Dr Dangs Lab, New Delhi; 7Biochemistry Division,
Indian Council of Medical Research, National Institute of
Nutrition, Hyderabad; 8Endocrinology, Sanjay Gandhi Post
Graduate Institute, Lucknow; 9Homeopathy, BR Sur Homeopathic
college, New Delhi.
Correspondence to: Major General RK Marwaha, President,
Society for Endocrine Health Care of Elderly, Adolescents
and Children (SEHEAC), Flat no. 17, Gautam Apartments,
Gautam Nagar, New Delhi 110 049.
Email:
marwaha_ramank@hotmail.com
Received: August 10, 2020;
Initial review: September 16, 2020;
Accepted: February 16, 2021.
Trial Registration: CTRI/2019/09/021073
Published online: July 23, 2021; PII:
S097475591600353
|
Objective: To evaluate the efficacy
of daily supplementation of 200 mL milk fortified with 240
IU of vitamin D2 (ergocalciferol).
Design: Double-blind randomized
controlled trial.
Settings: School-based study
in Delhi between October and December, 2019.
Participants: 235 healthy children
aged 10-14 years.
Intervention: Daily supplementation
of 200 mL milk fortified with 240 IU of ergocalciferol in
intervention group (n=119) and 200 mL of plain milk
in control group (n =116) for 3 months.
Outcome Measures: Change in serum 25
hydroxy vitamin D (25(OH)D), parathyroid hormone (PTH), bone
formation and resorption markers, and urinary calcium
creatinine ratio (U-Ca/CrR).
Results: The mean (SD) baseline serum
25(OH) D level in control and fortification groups was 11.9
(3.8) and 11.4 (3.6) ng/mL (P=0.23), respectively.
The serum 25(OH)D levels did not increase post-intervention
with the dose used for fortification, but were significantly
higher in intervention group as compared to control group
[10.8 (3.4) vs 6.7 (3.5) ng/mL; P<0.001]. A
higher proportion of secondary hyperparathyroidism was
observed post-intervention in control (39%) than in
intervention group (13.3%); P<0.001. Serum carboxy-terminal
telopeptide levels were similar in both groups but the serum
procollagen type1 N-terminal propeptide levels were higher
in the control than intervention group (P<0.007),
following supplementation.
Conclusion: Supplementation of milk
fortified with approximately 240 IU vitamin D2 for three
months did not achieve sufficient serum 25(OH)D levels in
Indian children with vitamin D deficiency during winter.
Keywords: Bone health, Deficiency, Food
fortification, Secondary hyperparathyroidism.
|
Optimum calcium and
vitamin D intake during childhood and adolescence helps
achieve peak bone mass which acts as a safeguard against
osteoporotic fractures later [1]. Consequences on overall
health require a population based approach for prevention of
vitamin D deficiency like food fortification [2], as
therapeutic supplementation throughout life is not
practical. A recent meta-analysis of the effects of vitamin
D fortification showed good efficacy of fortified dairy
products to increase vitamin D levels [2]. At present,
systematic voluntary or mandatory fortification of milk and
milk products is being undertaken only in few countries like
Finland, Norway, Sweden, Canada and USA [3].
In view of vitamin D deficiency being a
serious public health problem, Food Safety and Standards
Authority of India (FSSAI) issued instructions for voluntary
fortification of milk and oil with vitamin A and D2 to
provide approximately one-third (200-300 IU/L) of the
recommended daily dietary allowance [4]. However, the
adequacy and efficacy of these doses of vitamin D2 need to
be assessed in children.
We, therefore, undertook a double-blind
randomized controlled trial in healthy school children to
evaluate the efficacy of daily supplementation of 200 mL
fortified milk (approximately 240 IU of vitamin D2) on the
serum vitamin D levels. The secondary objectives included
effect of this intervention on serum levels of calcium,
parathyroid hormone, alkaline phosphatase and bone markers.
METHODS
This randomized double-blind parallel
placebo controlled study was conducted from October 1, 2019
(autumn) to December 30, 2019 (winter). The study protocol
was approved by the Institutional Ethics Committee and the
trial was registered prospectively at the clinical trial
registry of India. Apparently healthy school children, aged
10-14 years, who consented were recruited from two fee-
paying schools in Delhi following approval from the school
management. Written consent from parents of eligible
children and written assent from children was solicited.
Children with clinical features of rickets, history of any
chronic systemic illness, renal stones, history of milk
allergy, intake of vitamin D in last six months in doses
exceeding 600 IU/day or if consuming drugs like steroids,
anti-tubercular/anti-epileptic drugs were excluded.
Block randomization with varying block
size of 2 or 4 was used within each school to allocate the
children into fortified and control arm, respectively using
the computer-generated randomization list. Allocation
concealment was done using opaque envelopes which were
prepared by a person other than investigators. Participants
were assigned to one of the two groups as per the code by
the respective class teachers. The participants and care
providers were blinded to the randomization. The teachers
knew the codes as group A and B but did not know whether
milk in the respective group was fortified or not.
Two sets of strawberry flavored
ultra-heat treated toned milk in sterilized and homogenized
200 mL tetra packs were provided in the month of September,
2019 for the study by Mother Dairy Fruit and Vegetable Pvt.
Ltd, a licensed and registered firm by FSSAI. First set was
provided with 1200 IU of vitamin D2 per litre of milk
(approximately 240 IU of vitamin D2 in each tetra pack)
whereas other set was without fortification. The tetra packs
were similar in appearance, odor and taste with labels known
only to the manufacturers. The shelf life of tetra packs
containing fortified and unfortified milk was 120 days at
normal ambient temperatures, and were required to be kept in
cool, dry and pest free ambience. Samples of milk were
collected randomly at the time of production and after
completion of the study for stability and estimated by LC-MS
method (AOAC 2016.05).
Intervention group received fortified
milk whereas control group received unfortified milk for 3
months. Daily supplementation for 6 days a week was carried
out at schools under the supervision of teachers and
investigating staff. Tetra packs were provided to the
parents every month for Sundays and planned holidays.
Parents were advised to collect the tetra packes from the
school for unplanned holidays. A Whatsapp group was created
by each teacher with parents and chief co-ordinator for
day-to-day communication, monitoring and ensuring compliance
during planned and unplanned holidays.
Brief history and clinical examination
including anthropometry were performed. Heights were
measured to nearest 0.1 centimeter with portable Holtain
stadiometer (Holtain Inc.) with the child positioned in the
Frankfurt plane. Weights were measured to nearest 0.1 kg
with the digital weighing machine. The weighing scale and
stadiometer were calibrated using the standard weight and
height, respectively. Children were advised against any
change in lifestyle during the study period. Two day (one
working day and one holiday) 24-hour dietary recall method
and food frequency questionnaire were used to gather data on
dietary pattern and nutrient intake at baseline. The
consumption of calcium and vitamin D rich foods, and amount
and type of milk and oil consumed (fortified or not) were
also recorded. The household measures used for data
collection were standardized in the laboratory to obtain the
actual weight of raw foods going into each preparation.
Subsequently the data on food consumption in household
measures were converted into raw ingredients. The nutrient
intakes were then obtained by using the Diet Cal software
[5]. No dietary counselling was provided during the study
period.
Blood samples were collected in the
fasting state between 8-9 AM at baseline and after three
months (end-line). They were centrifuged and serum separated
into aliquots at the study site and transported in dry ice
to the laboratory. Serum calcium, phosphate, alkaline
phos-phatase (ALP), 25-hydroxy vitamin D [25(OH)D],
parathyroid hormone (PTH) and spot urinary calcium
creatinine ratio (U-Ca/CrR) were estimated the next day. Two
aliquots were frozen -700C
for estimation of bone markers. Serum 25(OH)D was estimated
by chemilu-minescence (DiaSorin Inc.) and PTH by
electro-chemiluminescence method (Roche Diagnostics). Intra
and inter-assay coefficient of variation was 3.5% and 5% for
serum 25(OH)D and 2.4% and 3.6% for PTH. Serum 25(OH)D level
of <20 ng/mL was defined as insufficiency and <12 ng/mL as
vitamin D deficiency (VDD) [6]. Secondary
hyperparathyroidism was defined as PTH >65 pg/mL. Serum
calcium, phosphate and ALP were estimated by auto-analyzer
Cobas C-501 (Roche Diagnostics). Serum bone markers viz.,
C-terminal crosslinked telopeptide of type 1 collagen
(CTx-1) and propeptide of N-terminal of type 1 collagen
(PINP) were measured by Elecsys 2010 based on principle of
electrochemimmunoassay. U-Ca/CrR was estimated using Cobas
C-3 (Roche Diagnostics) with a level >0.21 suggestive of
hypercalciuria [7].
The sample size was calculated assuming
baseline mean (SD) of serum 25(OH)D of 11.7 (5.36) ng/mL in
both groups. Expecting no change in control group and an
increase of 3 ng/mL in serum 25(OH)D levels after 3 months
of supplementation with combined SD of 3 ng/mL [8], the
estimated sample size was 68 per group. The assumed alpha
error and power were 5% and 90%, respectively. The total
number of subjects required for the study with 20% drop out
rate was 90 per group.
Statistical analysis: Continuous
variables were summarized as mean (SD) (normally
distributed) or median (Q1,Q3) (non-normally distributed).
Categorical variables were presented as proportions.
Baseline characteristics were compared between the groups
using unpaired t-test or Chi-square test as
appropriate. Intention-to-treat (ITT) analysis was done for
effect on serum 25(OH)D and serum PTH levels and per
protocol (PP) analysis was carried out for other biochemical
variables. All the outcomes were compared between the groups
using unpaired t-test/ Wilcoxon rank sum test and
within the group (from baseline to 3-months) using paired
t-test/Wilcoxon signed rank test. The results were
presented as difference and 95% confidence interval. P
value less than 0.05 was considered statistically
significant.
RESULTS
The flow of the study participants is
shown in Fig. 1. Table I shows the baseline
demographic characteristics. No child from either of the
group reported any discomfort or gastrointestinal
side-effects after consuming milk. Samples of milk were
collected randomly at the time of production and on
completion of the study for stability, which showed serum
25(OH)D levels of 236 IU/200 mL and 221 IU/200 mL,
respectively indicating <10% variation.
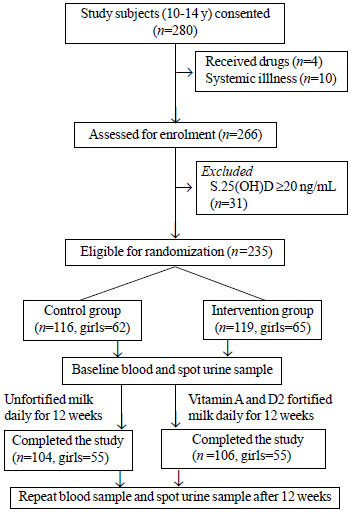 |
|
Fig. 1 CONSORT flow diagram
for the study.
|
Table I Baseline Characteristics of the Study Population
| Parameter |
Unfortified group
|
Fortified group |
|
(n=119) |
(n=116) |
| Age, y |
10.4 (0.8) |
10.3 (0.5) |
| Body mass index, kg/m2 |
16.8 (3.2) |
16.7 (3.3) |
|
Calcium, mg/dL
|
9.97 (0.3) |
10.01 (0.3) |
|
Phosphorus, mg/dL
|
5.02 (0.49) |
5.01 (0.46) |
|
ALP, IU/mL
|
258.83 (72.3) |
253.49 (62.3) |
|
25(OH)D, ng/mL
|
11.97 (3.79 ) |
11.42 (3.63) |
|
PTH, pg/mLa |
45.8 (35.5, 60.0) |
52.3 (35.3, 61.6) |
|
CTX, pg/mL
|
1705.7 (483.2) |
1685.4 (400.7) |
|
PINP, ug/dL
|
655.3 (203.4) |
679.9 (211.4) |
|
Urine Ca: Cr ratioa |
0.04 (0.01, 0.1) |
0.05 (0.03, 0.09) |
|
Data expressed as mean (SD) or amedian (IQR);
ALP-alkaline phosphatase, 25(OH)D- 25 hydroxy
vitamin D, PTH- parathyroid hormone, CTX- C-terminal
crosslinked telopeptide of type 1 collagen, PINP-
propeptide of N-terminal of type 1 collagen, Ca:Cr-
calcium: creatinine P>0.05 for all variables |
The baseline serum 25(OH)D levels were
similar in the control and fortification groups (Table I),
with significantly higher end-line levels in the
intervention than in the control group as shown in Table
II. The number of subjects with vitamin D deficiency and
insufficiency at baseline were 70 (60%) and 46 (40%) in the
control group and 65 (54.6%) and 54 (45.4%) in the
intervention group, respectively (P=0.37). Vitamin D
deficiency, insufficiency and sufficiency after three months
were noted in 101 (97%), 3 (3%), 0 in control and 74 (70%),
31 (29%), 1 (1%) in the intervention group, respectively (P<0.001).
The median (Q1,Q3) percentage rise in serum 25(OH) D was
significantly higher among subjects with serum 25 (OH)D
levels <12 ng/mL [n=58, 10.64 (–48.71, –5.66)]
than those with levels >12 ng/mL [n=48, -20.41
(–34.36, –7.67)] in the intervention group (P<0.001).
There was poor correlation between serum 25(OH)D and gender
or BMI (P>0.05).
Table II Biochemical Parameters After Intervention in Unfortified and Fortified Groups
| Parameter |
Unfortified group
|
Fortified group |
P |
|
(n=104) |
(n=106) |
value |
|
Calcium, mg/dL
|
9.88 (0.3)c |
9.98 (0.3) |
0.01 |
|
Phosphorus, mg/dL
|
4.97 (0.45) |
4.96 (0.42) |
0.80 |
|
ALP, IU/mL
|
273.24 (75.5)c |
251.32 (57.7) |
0.02 |
|
25 (OH)D,ng/mLb
|
6.73 (3.5)a |
10.81 (3.5) |
<0.001 |
|
PTH, pg/mLb,a
|
52.6 (38.8,75.2)c |
46.5 (32.2,58.8)c |
0.007 |
|
CTX, pg/mL
|
1023.22 (317.7)c |
982.42 (304)c |
0.35 |
|
PINP, ug/dL
|
736.33 (201.4)c |
657.32 (216.5) |
0.007 |
|
Urine Ca:Cr ratioa |
0.04
(0.02,0.1) |
0.04
(0.02,0.08) |
0.86 |
|
All parameters are serum unless stated. cP<0.05 for
intragroup comparison from baseline to
post-intervention value. Data expressed as mean (SD)
or amedian (Q1, Q3). bn=116 and 119 in unfortified
and fortified group, respectively (intention to
treat analysis). ALP-alkaline phosphatase,
25(OH)D-25 hydroxy vitamin D, PTH-parathyroid
hormone, CTX-C-terminal crosslinked telopeptide of
type 1 collagen, PINP-propeptide of N-terminal of
type 1 collagen, Ca:Cr-calcium: creatinine. |
The prevalence of secondary
hyperthyroidism increased from 18.1% to 39% (P<0.001)
in the control group and decreased from 22.7% to 13.3% (P<0.001)
in the intervention group, with significant inter-group
difference (P<0.001). An inverse correlation was
observed between serum 25(OH)D and PTH both at baseline in
control (r=-0.23, P=0.01) and intervention
groups (r=-0.29, P=0.001) and following
supplemen-tations in both groups (r= -0.23, P=0.01);
(r= -0.29, P=0.001), respectively. No subject
in either group developed hypercalciuria following
supplementation.
The overall energy intakes were less
(70.6%) than the RDA, with adequate protein intakes in the
study group. The mean (SD) intake of calcium in intervention
and control groups was 655.3 (224.1) and 617 (240.3) mg/day
with dairy calcium contributing an intake of 57.4% and 58.4%
in both groups. The vitamin D intake through fortified foods
ranged from 17-97 IU/day in both the groups.
DISCUSSION
The present study demonstrated higher
serum 25(OH)D levels following consumption of vitamin D2
fortified milk (240 IU/200 mL) for a period of 3 months as
against consumption of unfortified milk, with significant
decrease in secondary hyperparathyroidism.
Several studies in children have
similarly observed higher serum 25(OH)D levels after
consumption of vitamin D fortified milk than unfortified or
no milk at all [2,3, 9]. Higher serum 25(OH)D levels were
seen in children who consumed at least 450 mL/day of vitamin
D fortified milk than those who drank < 300 mL/day after
adjusting for age and sex [9].
The serum 25(OH)D levels did not increase
above the baseline in the fortified group with the current
levels of fortification; however, the decline was lesser
than the unfortified group. This suggested that 240 IU of
additional vitamin D2 through fortified milk for 3 months
was not adequate during harsh winter months with high
atmospheric pollution recorded in Delhi during the study
period, when the availability of UVB rays was low [10,11].
Inadequate synthesis of vitamin D3 during winter months in
children has been similarly reported earlier [12].
Vitamin D3 has higher efficacy than D2 in
raising serum 25(OH)D levels [13-15]. However, whether
fortification with D3 instead of D2 would have resulted in
higher serum 25(OH)D levels is debatable and outside the
purview of the current study. A rise in serum 25(OH)D levels
was reported earlier with almost similar dose of 200 IU of
D3 supplementation for 12 months [16], unlike no change
observed in another study after 11 weeks of supplementation
in healthy adolescents with baseline vitamin D sufficiency
[17]. These contrasting observations could be because of
varying baseline 25(OH)D levels, duration of intervention,
vitamin D preparations and modes of supplementation. The
rise in serum 25(OH)D was significantly higher in those with
lower baseline serum 25(OH)D levels in the present study, as
also reported earlier [8,16,18,19].
A significant reduction in serum PTH
levels and secondary hyperparathyroidism in the intervention
group suggests the role of even a small amount of vitamin D
administered with calcium in reducing negative consequences
on bone mineral metabolism. Similarly, inverse correlation
between serum 25(OH)D and PTH levels have been documented
earlier [16,18,20].
Earlier studies have not reported a
significant effect on either bone formation or resorption
markers [21,22]; however, a decrease in resorption markers
like serum CTx and urinary deoxypyridiniline is reported
following vitamin D supplementation [23,24]. In the present
study, a significant decline in serum CTx levels in both the
groups with no appreciable inter group differences, could be
due to the additional calcium provided through milk. Similar
observation was also reported in healthy premenopausal women
following calcium supple-mentation [25]. No significant
change in serum PINP levels following supplementation in the
intervention group concurs with earlier studies where bone
specific ALP, serum osteocalcin and serum PINP remained the
same [21,22,24]. These variations in bone markers following
vitamin D supplementation may be due to differences in age
of subjects, doses of vitamin D, duration of supplementation
and baseline serum 25(OH)D etc. Spot U-Ca/CrR measured
revealed no significant difference in the median U-Ca/Cr
ratios at baseline and follow-up.
The main strength of the study was the
evaluation of the efficacy of supplementing vitamin D2
fortified milk in children. The study; however, had
limitations like absence of data on environmental pollution
and sunlight exposure, lack of comparison with D3 fortified
milk, and inability to carry out 24-hour urinary calcium
excretion for definite diagnosis of hypercalciuria.
To conclude, supplementation of milk
fortified with 240 IU Vitamin D2 for 12 weeks is not
adequate to achieve vitamin D sufficiency, though it does
reduce the decline in serum 25(OH)D levels during winter in
prepubertal Indian children with vitamin D insufficiency.
Acknowledgements: Ms Pamela
Marwaha for complete supervision of the project and Akanksha
Chand for collection of dietary data. Mother Dairy Fruit and
Vegetables Private Ltd for providing Tetra packs of milk at
concessional rate for the study.
Ethics clearance:
Institutional Ethics Committee, Maulana Azad Medical
College; No. F.1/MAMC/IEC/(68/03/2019)/No 153, dated 09
August, 2019.
Contributors: RKM:
conceptualizing and designing the study, clinical evaluation
and preparation of the manuscript; AD,VD: clinical
evaluation, analysis of data and preparation of manu-script;
SY: designing the study and preparation of manuscript; SP:
designing of dietary proforma, analysis of dietary data,
manuscript preparation; AD: (Arjun Dang) Biochemical and
hormonal evaluation, manuscript review; KV: sample size
calculation and statistical analysis, manuscript
preparation; PR: conceptualization and preparation of
manuscript; SG: analysis of bone markers, manuscript review;
AN: sample and data collection and data entry, manuscript
review. All authors have read and approved the final
manuscript.
Funding: The Initiative Nutrition of
India (TINI) to Society for Endocrine Health Care of
Elderly, Adolescents and Children (SEHEAC) (RKM).
Competing interest: None stated.
|
WHAT IS ALREADY KNOWN?
•
Fortification of milk with vitamin D is an
effective strategy for preventing vitamin D
deficiency.
WHAT THIS STUDY ADDS?
•
Consumption of 200 mL of fortified milk
(containing 240 IU vitamin D2) for 12 weeks is
inadequate in preventing vitamin D deficiency in
school children from Delhi during winter season.
|
REFERENCES
1. Weaver CM, Gordon CM, Janz KF, et
al. The National Osteoporosis Foundation’s Position
Statement on Peak Bone Mass Development and Life Style
Factors: A Systemic Review and Implementation
Recommendations. Osteoporos Int. 2016;27:1281-386.
2. Black LJ, Seamans KM, Cashman KD,
Kiely M. An updated systematic review and meta-analysis
of the efficacy of vitamin D food fortification. J Nutr.
2012;142:1102 8.
3. Itkonen ST, Erkkola M,
Lamberg-Allardt CJE. Vitamin D fortification of fluid
milk products and their contribution to vitamin D intake
and vitamin D status in observational studies - A
review. Nutrients. 2018;10:1054.
4. Food Safety and Standards
Authority of India (FSSAI). Ministry of Health and
Family Welfare, Government of India. Standards of
fortification of standard foods. Notification dated 2
Aug 2018. Accessed on 09 March, 2020. Available from:
https://archive.fssai.gov.in/home/fss-legislation/notifications/gazette-notification.html
5. Narasinga Rao BS, Sivakumar
S.Nutrient Requirements and Recommended Dietary
Allowances for Indians. Indian Council of Medical
Research; 2010.
6. From Indian Academy of Pediatrics
‘Guideline for Vitamin D and Calcium in Children’
Committee, Khadilkar A, Khadilkar V, Chinappa J, et al.
Prevention and Treatment of Vitamin D and Calcium
Deficiency in Children and Adolescents: Indian Academy
of Pediatrics (IAP) guidelines. Indian Pediatr.
2017;54:567-73.
7. Metz MP. Determining urinary
calcium/ creatinine cut offs for the pediatric
population using published data. Ann Clin Biochem.
2006;43:398-401.
8. Khadgawat R, Marwaha RK, Garg MK,
et al. Impact of vitamin D fortified milk
supplementation on vitamin D status of healthy school
children aged 10-14 years [published correction appears
in Osteoporos Int. 2014;25:1655]. Osteoporos Int.
2013;24:2335 43.
9. Soininen S, Eloranta AM, Lindi V,
et al. Determinants of serum 25-hydroxyvitamin D
concentration in Finnish children: The Physical Activity
and Nutrition in Children (PANIC) study. Br J Nutr.
2016;115:1080-91.
10. Weather online- Delhi, India.
Accessed March 12, 2020. Available from: https://www.weatheronline.co.uk/
weather /maps/city
11. Delhi records poorest air quality
in 3 years (2019). Accessed January 12, 2020. Available
From:https://economic
times.indiatimes.com/news/politics-and-nation/delhi-records-poorest-air-quality-in-3-years/articleshow/71880776.cms?utm_source=contentofinterest&utm_
medium=text&utm_campaign=cppst
12. Marwaha RK, Yenamandra VK,
Sreenivas V, et al. Regional and seasonal variations in
Ultraviolet B irradiation and vitamin D synthesis in
India. Osteoporosis Int. 2016;27:1611-7.
13. Tripkovic L, Wilson LR, Jhonson
S, et al. Daily supplementation with 15ug vitamin D2
compared with vitamin D3 to increase wintertime
25-hydroxyvitamin D status in healthy South Asian and
white European women: A 12-wk randomized placebo
controlled food fortification trial. Am J Clin Nutr.
2017;106:481-90.
14. Heaney RP, Recker RR, Grote J,
Horst RL, Armas LAG. Vitamin D3 is more potent than
vitamin D2 in humans. J Clin Endocrinol Metab.
2011;96:E447-52.
15. Tripkovic L, Lambert H, Hart K,
et al. Comparison of vitamin D2 and vitamin D3
supplementation in raising serum 25-hydroxyvitamin D
status: A systematic review and meta-analysis. Am J Clin
Nutr. 2012;95:1357-64.
16. Al-Shaar L, Mneimneh R, Nabulsi,
Maaloof J, El-Hajj Fuleihan G. Vitamin D3 dose
requirement to raise 25-hydroxy D to desirable levels in
adolescents: Results from a randomized controlled trial.
J Bone Mineral Res. 2014;29: 944-51.
17. Putman MS, Pitts SA, Milliren CE,
Feldman HA, Reinold K, Gordon CM. A randomized clinical
trial of vitamin D supplementation in healthy
adolescents. J Adolesc Health. 2013; 52:592-8.
18. Marwaha RK, Garg MK, Sethuraman
G, et al. Impact of three daily doses of vitamin D3
supplementation in healthy school children and
adolescents from North India: A single- blind
prospective randomized clinical trial. Br J Nutr.
2019;121:538-48.
19. Jääskeläinen T, Itkonen ST,
Lundqvist A, et al. The positive impact of general
vitamin D food fortification policy on vitamin D status
in a representative adult Finnish population: evidence
from an 11-y follow up based on standardized
25-hydroxyvitamin D data. Am J Clin Nutr.
2017;105:1512-20.
20. Marwaha RK, Mithal A, Bhari N, et
al. Supplementation with three different daily doses of
vitamin D3 in healthy pre-pubertal school girls: A
cluster randomized trial. Indian Pediatr. 2018;55:951-6.
21. Hill KM, Laing EM, Hausman DB, et
al. Bone turnover is not influenced by serum
25-hydroxyvitamin D in pubertal healthy black and white
children. Bone. 2012;51:795-9.
22. Mølgaard C, Larnkjaer A, Cashman
KD, Lamberg-Allardt C, Jakobsen J, Michaelsen KF. Does
vitamin D supple-mentation of healthy Danish Caucasian
girls affect bone turnover and bone mineralization?
Bone. 2010;46:432-9.
23. Marwaha RK, Garg MK, Mithal A,
Gupta S, Shukla M, Chadha A. Effect of vitamin D
supplementation on bone turnover markers in children and
adolescents from North India. Indian J Endocrinol Metab.
2019;23:27-34.
24. Viljakainen HT, Natri AM,
Kärkkäinen M, et al. A positive dose-response effect of
vitamin D supplementation on site-specific bone mineral
augmentation in adolescent girls: A double-blinded
randomized placebo-controlled 1-year intervention. J
Bone Miner Res. 2006; 21:836-44.
25. Ferrar L, van der Hee RM, Berry M, et al. Effects of
calcium-fortified ice cream on markers of bone health.
Osteoporos Int. 2011;22:2721-31.
|
|
|
 |
|

