|
|
|
Indian Pediatr 2019;56: 741-744 |
 |
Hepatitis A Virus-related Pediatric Liver
Disease Burden and its Significance in the Indian Subcontinent
|
|
Vikrant Sood 1,
Bikrant Bihari Lal1,
Ekta Gupta2,
Rajeev Khanna1,
Manish K Siloliya1
and Seema Alam1
From Departments of 1Pediatric Hepatology
and 2Clinical Virology, Institute of Liver and Biliary
Sciences,
Vasant Kunj, New Delhi, India.
Correspondence to: Dr Seema Alam, Professor and Head,
Department of Pediatric Hepatology, Institute of Liver and Biliary
Sciences, Vasant Kunj, New Delhi 110 070, India.
Email: seema_alam@hotmail.com
Received: November 05, 2018;
Initial review: April 15, 2019;
Accepted: June 20, 2019.
|
|
Objectives: To study the
Hepatitis A virus (HAV) infection-related pediatric liver disease
burden. Methods: Hospital records of 431 children (age <18
y) diagnosed to be suffering from acute HAV infection during 2011 to
2018 were extracted and analyzed. Additionally, a seroprevalence study
was done on 2599 participants (696 children and 1903 adults).
Results: HAV infection accounted for about half (48.6% of acute
hepatitis and 46.5% (92/198) of acute liver failure cases) of all acute
onset icteric illness, with significant morbidity and mortality. As per
seroprevalence data, 16.2% of children between 10-18 years of age, and
10.3% of adults aged 18-30 years remained susceptible to HAV infection.
Conclusion: HAV infection is the major contributor the overall
pediatric liver disease burden. A significant proportion of subjects
remain susceptible to HAV infection even after 10 years of age.
Population-based studies are required to further delineate the
epidemiology of HAV infection in India for deciding introduction of HAV
vaccine in the national immunization schedule.
Keywords: Acute viral hepatitis, Hepatitis A
infection, Seroprevalence rate, Vaccination.
|
|
H
epatitis A virus infection is
the commonest
cause of pediatric liver disease in India, with
severity varying from uncomplicated
subclinical/clinical acute viral hepatitis (AVH) to acute or
acute-on-chronic liver failure. In Indian subcontinent, proportion of
overall AVH, acute liver failure, and acute-on-chronic liver failure
cases attributed to HAV infection is around 70-85%, 40-60%, and 10-40%,
respectively [1-3]. This highlights the significance of HAV infection,
especially when it is one of the only few vaccine preventable hepatic
diseases. Universal immunisation against HAV in children in India is
still controversial with limited national epidemiological data on HAV
epidemiology [4]. Thus, we thus planned this hospital-based study to
assess the HAV-related pediatric liver disease burden in a high volume
tertiary-care referral centre, which may serve as a template for future
population-based studies on hepatitis A epidemology and vaccination
policy.
Methods
A retrospective review, from electronic case records,
was done after Institutional Ethics Committee approval. We included all
pediatric patients (<18 years of age at presentation) who presented with
acute onset icteric illness with suspected viral hepatitis from year
2011-2018. These included patients with AVH, acute liver failure, and
acute-on-chronic liver failure, defined as per standard definitions
[5,6]. Previous publications from the same centre included a proportion
of these subjects [2,3]. All patients who tested positive for IgM
antibody for HAV using chemiluminescent microparticle immunoassay (CMIA)
technology (Abbott Laboratories, IL, USA) were included in the present
study (Fig. 1). These patients were divided into three
groups; Group I: children with AVH, Group II: Acute liver failure, and
Group III: children with Acute-on-chronic liver failure.
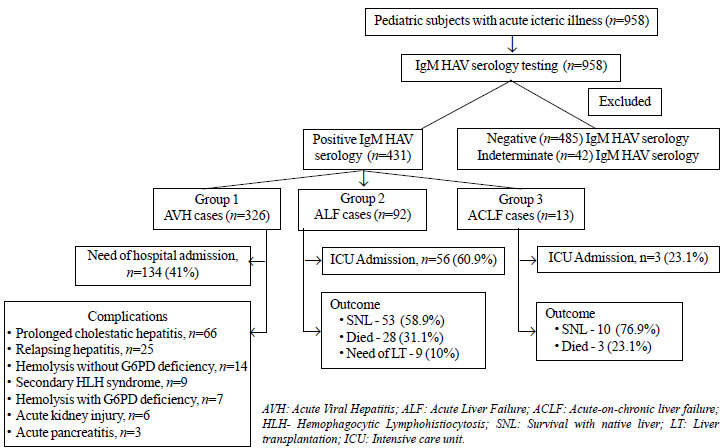 |
|
Fig. 1 Outcomes of the pediatric
subjects with hepatitis A virus (HAV) infection.
|
To assess the baseline seroprevalence for protective
antibody against HAV, all subjects who were tested for total anti-HAV
antibody (IgG, by CMIA technology; Abbott Laboratories, IL, USA) during
the same time period were also included. This testing is the standard
practice in our institute in those not previously vaccinated with HAV
vaccine, or in those with doubtful vaccination history/without relevant
vaccination records, before prescribing HAV vaccination. Prescription of
HAV vaccine is included in our routine clinical practice as per
institutional policy. Patients who tested positive for this antibody
were diagnosed having either past exposure to HAV infection, or prior
(but unknown) vaccination against HAV.
Results
A total of 958 children and adolescents presented
with acute onset icteric illness during the study period; out of these,
431 (44.9 %) were diagnosed as having acute HAV infection. Median (IQR)
age of the patients was 11 (6-14) years. Male:female ratio was 2.5:1.
Age-wise distribution of total subjects was: <5 years of age: n=67,
³5 to <10
years of age: n=120, and ³10
to <18 years of age: n=244. As shown in Fig. 1,
majority of these subjects were in group I i.e. AVH group (group
1: 326 or 48.6% out of total 671 AVH cases); of these, 41.1% required
hospital admission due to varied reasons including associated
complications. Overall, median (IQR) length of hospital stay was 3.5
(3-5) days.
Acute HAV infection contributed to 92 (46.5%) of the
total 198 cases of acute liver failure (Group II). The median (IQR) age
of these children was 9.5 (7-11) years. The median (IQR) length of
hospitalization was 10 (7-13) days. Sixty (65.2%) had hepatic
encephalopathy grade 3-4 at admission with median (IQR) jaundice to
encephalo-pathy interval of 3 (2-16) days. Intensive care unit admission
was required in 56 (60.9%) of these subjects with median ICU stay of 5
(IQR 2-9) days. Fifty-one of them required mechanical ventilation for
raised intracranial pressure [median (IQR) duration: 5 (2-7.5) days].
Overall, 53 (58.9%) survived with their native liver,
28 (31.1%) died, and 9 (10%) underwent liver transplantation.
Of the total patients with acute-on-chronic liver
failure (n=89), HAV infection contributed to 14.6% (n=13)
of such subjects (Group III). The median age (range) of presentation was
6.9 (3-12) years. All had ascites and encephalopathy. Grade 3-4 hepatic
encephalopathy was present in 3 (23.1%). Median length of hospital stay
was 16 (range 2-60) days. ICU admission was required in 3 (23.1%) of
these subjects with median ICU stay of 5 days; of these cases, 2
subjects (15.4%) required mechanical ventilation. Three (23.1%) children
died and the rest survived with their native liver.
Seroprevalence results: For the seroprevalence
data, a total of 696 pediatric subjects underwent testing for IgG
antibody against HAV infection. These subjects were divided into three
age groups i.e. <5 years of age (n=66),
³5 to <10 years of
age (n=247), and ³10
to <18 years of age (n=383). Data revealed that 27.3%, 21.1% and
16.2% subjects were negative for the protective IgG antibody in these
three age groups, respectively. Similar analysis was done in a total of
1903 adult subjects. These subjects were divided into four age groups
i.e. ³18
to <30 years of age (n=380),
³30 to <40 years of age (n=437),
³40 to <50 years of
age (n=527), and >50 years of age (n=559). Of these,
10.3%, 0.7%, 0.6% and 0% subjects were still negative for the protective
IgG antibody in these four age groups, respectively.
Discussion
Despite being limited by its single center/tertiary
care hospital-based and retrospective nature, the present study
highlights the burden of HAV-related pediatric liver disease. HAV
infection accounted for about half of all acute onset icteric illness
and acute liver failure cases, and was associated with significant
morbidity and mortality. Even after 10 years of age, almost 10-15%
subjects remained susceptible to HAV infection.
Hepatitis A as the most common cause of pediatric AVH
and acute liver failure has been previously also highlighted [1,2]. The
currently available literature highlights the fact that HAV infection
has now assumed a more significant role with increasing disease related
burden, owing to tremendous success in the vaccination strategies for
other diseases. As per latest available national estimates, overall
44,663 cases of HAV infection were detected between 2011-2013 [7]. This
burden is reportedly much higher than the current case load (per year)
for all other vaccine preventable diseases in India, except Pertussis
(in year 2013) [8]. As per global estimates of mortality, HAV infection
ranked sixth amongst infectious (vaccine preventable) causes of
worldwide mortality (ahead of Pertussis, Tetanus, Varicella and
Diphtheria) [9]. The same study also revealed that HAV infection is the
only infectious disease entity with increasing mortality risks over the
years, while risks have decreased for others.
Recent literature has shown that India is now
witnessing an epidemiological transition from high to intermediate
endemicity owing to rapid (but unequal) development and improving
standards of hygiene, as highlighted by the progressively decreasing
age-related seroprevalence rates, as in the present study [4,10-12].
This has created multiple heterogenous pockets in the country where
there are large population groups who still remain susceptible to HAV
infection. This shift or transition is a critical phase, which if
compounded by inadequate preventive HAV vaccination program (as is the
current scenario in India), may serve to transform the scenario from an
‘endemic’ to ‘epidemic’ pattern [4]. This could lead to repeated
outbreaks of HAV related disease in such susceptible populations and
also paradoxical increase in the incidence, morbidity and mortality due
to HAV infection (beyond pediatric age group), as has happened in India
and in other countries (Web Fig. 1; concept diagram)
[13-15]. The impact of introduction of universal HAV vaccine has been
highlighted from several countries, including developing countries like
Argentina [16-21]. Along with remarkable reduction in incidence of
symptomatic infection (across all age groups; with percentage decline in
HAV incidence varying from 76 to 90 % after introduction of vaccine),
they could also document the fact that the monetary benefits due to
reduced medical expenditure far exceeded the immunization costs [17,
21].
World Health Organization recommends that countries
undergoing transition from high to intermediate HAV endemicity should
consider introduction of large-scale HAV vaccination [22]. However, this
decision must be based on actual national seroprevalence data (using
seroepidemiologic surveys and intensive disease surveillance), along
with indigenous cost-effectiveness analyses [4]. In this context, the
present study highlights the significance of HAV-related pediatric
disease burden in the region, and underlines the unmet need of further
population-based large studies to further elucidate the epidemiology of
HAV infection in India, in order to decide/prioritize the inclusion of
HAV vaccination in the national immunization schedule.
Contributors: VS, BBL, RK and MKS contributed to
data collection and writing the initial draft of manuscript; EG, RK and
SA conceptualized the study, and supervised the editing and revision of
manuscript. All authors approved the final version of manuscript, and
are accountable for all aspects related to the study.
Funding: None; Competing interest: None
stated.
|
What This Study Adds?
• Hepatitis A infection contributed to about
half of cases of acute icteric illness in children reporting to
our center.
• About 10-15% of population remain susceptible to HAV
infection even after 10 years of age.
|
References
1. Yachha SK, Goel A, Khanna V, Poddar U, Srivastava
A, Singh U. Ascitic form of sporadic acute viral hepatitis in children:
A distinct entity for recognition. J Pediatr Gastroenterol Nutr. 2010;50:184-7.
2. Alam S, Khanna R, Sood V, Lal BB, Rawat D. Profile
and outcome of first 109 cases of paediatric acute liver failure at a
specialized paediatric liver unit in India. Liver Int. 2017;37:1508-14.
3. Alam S, Lal BB, Sood V, Rawat D. Pediatric
acute-on-chronic liver failure in a specialized liver unit: Prevalence,
profile, outcome and predictive factors. J Pediatr Gastroenterol Nutr.
2016;63:400-5.
4. Aggarwal R, Goel A. Hepatitis A: epidemiology in
resource-poor countries. Curr Opin Infect Dis. 2015;28: 488-96.
5. Squires RH Jr, Shneider BL, Bucuvalas J, Alonso
E, Sokol RJ, Narkewicz MR et al. Acute liver failure in children:
the first 348 patients in the pediatric acute liver failure study group.
J Pediatr. 2006;148:652-658.
6. Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al
Mahtab M, Rahman S, et al; APASL ACLF Research Consortium (AARC)
for APASL ACLF working Party. Acute-on-chronic Liver Failure:
Consensus Recommendations of the Asian Pacific Association for the Study
of the Liver (APASL): An Update. Hepatol Int. 2019 Jun 6. [Epub ahead of
print]
7. Morbidity and Mortality Weekly Report (MMWR):
Viral Hepatitis Surveillance—India, 2011–2013. Available from:
https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6428a3.htm. Accessed
September 7, 2018.
8. WHO vaccine-preventable diseases: monitoring
system. 2018 global summary. Available from: http://apps.who.int/immunization_monitoring/globalsummary/countries?
countrycriteria%5Bcountry%5D%5B%5D= IND&commit =OK. Accessed
September 9, 2018.
9. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K,
Aboyans V, et al. Global and regional mortality from 235 causes
of death for 20 age groups in 1990 and 2010: A systematic analysis for
the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128.
10. Franco E, Meleleo C, Serino L, Sorbara D, Zaratti
L. Hepatitis A: Epidemiology and prevention in developing
countries. World J Hepatol. 2012;4:68-73.
11. Gripenberga M, D’Corb NA, L’Azouc M, Marshd G,
Druellesc S, Nealon J. Changing sero-epidemiology of hepatitis A in
Asia-Pacific countries: A systematic review. Int J Infect Dis. 2018; 68:
13-7.
12. Arankalle V, Mitra M, Bhave S, Ghosh A,
Balasubra-manian S, Chatterjee S, et al. Changing epidemiology of
hepatitis A virus in Indian children. Vaccine: Development and Therapy.
2014;4:7-13.
13. Yao G. Clinical spectrum and natural history of
viral hepatitis A in a 1988 Shanghai epidemic. In: Hollinger FB,
Lemon SM, Margolis H, editors. Viral Hepatitis and Liver Disease:
Proceedings of the 1990 International Symposium. Baltimore, MD: Williams
and Wilkins; 1991. p. 76 80.
14. Arankalle VA, Sarada Devi KL, Lole KS, Shenoy
KT, Verma V, Haneephabi M. Molecular characterization of hepatitis A
virus from a large outbreak from Kerala, India. Indian J Med Res.
2006;123:760-9.
15. Rakesh P, Sherin D, Sankar H, Shaji M, Subhagan
S, Salila S. Investigating a community-wide outbreak of hepatitis A in
India. J Glob Infect Dis. 2014;6:59-64.
16. Vizzotti C, González J, Gentile A, Rearte A,
Ramonet M, Cañero-Velasco MC, et al. Impact of the single-dose
immunization strategy against hepatitis A in Argentina. Pediatr Infect
Dis J. 2014;33:84-8.
17. Vizzotti C, Pippo T, Uruena A, Altuna J, Palópoli
G, Hernández ML, et al. Economic analysis of the single-dose
immunization strategy against hepatitis A in Argentina. Vaccine.
2015;33:A227-32.
18. Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval
D. Incidence of hepatitis A in Israel following universal immunization
of toddlers. JAMA. 2005;294:202-10.
19. Chironna M, Prato R, Sallustio A, Martinelli D, Tafuri
S, Quarto M, et al. Hepatitis A in Puglia (South Italy) after 10
years of universal vaccination: need for strict monitoring and catch-up
vaccination. BMC Infect Dis. 2012; 12:271.
20. Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W,
Yuansheng H, et al. Hepatitis A surveillance and vaccine use in
China from 1990 through 2007. J Epidemiol. 2009;19:189-95.
21. Suwantika AA, Yegenoglu S, Riewpaiboon A, Tu HA,
Postma MJ. Economic evaluations of hepatitis A vaccination in middle
income countries. Expert Rev Vacc. 2013;12:1479-94.
22. No authors listed. WHO position paper on hepatitis A vaccines –
June 2012. Wkly Epidemiol Rec. 2012;87: 261-76.
|
|
|
 |
|

