|
|
|
Indian Pediatr 2019;56:735-740 |
 |
Predictive Value of
Vasoactive-inotropic Score for Mortality in Newborns Undergoing
Cardiac Surgery
|
|
Dilek Dilli 1,
Hasan Akduman1, Utku
Arman Orun2, Mehmet
Tasar3, Irfan
Tasoglu4, Seda
Aydogan1, Rumeysa
Citli1 and Sercan
Tak3
From the Departments of 1Neonatology,
2Pediatric Cardiology and 3Pediatric
Cardiovascular Surgery, Sami Ulus Maternity and Children Research and
Training Hospital; and 4Turkiye Yuksek Ihtisas Training and
Research Hospital, University of Health Sciences; Ankara,Turkey.
*Correspondence to: Dr Dilek Dilli, Sami Ulus Maternity and Children
Research and Training Hospital, Ankara/Turkey. Email:
[email protected]
Received: March 15, 2019;
Initial review: April 09, 2019;
Accepted: July 12, 2019.
|
Objective: Vasoactive-inotropic Score (VIS) was developed to
quantify the amount of inotropic support provided in the postoperative
period. We investigated the predictive value of (VIS) for mortality in
neonates with congenital heart disease (CHD). Study design:
Prospective cohort. Patients: 119 newborns who underwent cardiac
surgery. Setting: Tertiary NICU-CHD center of Ankara from
November 2016 to January 2019. Intervention/Measurement: VIS
values were calculated by a standard formula for the first 72
postoperative hours, and the maximum score was recorded. Primary
outcomes: Duration of mechanical ventilation, NICU length of stay,
and mortality. Results: At surgery, the median (IQR) age was 15 d
(9-31). The patients were divided into two groups according to
mortality; Group 1 (Non-survivors) (n=36) and Group 2 (Survivors)
(n=83). Higher VIS score was correlated to longer duration of
mechanical ventilation (P=0.009, r=0.33), and was higher
among patients who died (P=0.003). Area under the curve (AUC) was
0,83 (P<0.001, CI: 95% 0.7-0.9) for VIS to identify mortality. At
a cut-off value of 15.5, sensitivity and negative predictive values of
VIS for mortality were 73.6% and 85.3%, respectively. The higher VIS
(>15.5) was independently associated with increased odds for mortality
(OR: 8.1, 95% CI: 1.8-35.7, P=0.005). Conclusions: In
newborns with CHD, a higher VIS within 72 hours after cardiac surgery is
associated with increased duration of mechanical ventilation, and
mortality. VIS may be useful for prediction of mortality at early
postoperative period.
Keywords: Congenital heart disease, Outcome, Repair, Survival.
|
|
T
he incidence of congenital heart disease (CHD) in
the neonatal intensive care unit (NICU) has been reported as 3.7% in
term and 6.8% in premature infants [1]; in Turkey, the prevalence rate
is 7.7% [2]. Over the past three decades, significant technologic
advances have improved outcomes in neonatal cardiac surgery [3,5]. There
has been much research on the preoperative factors associated with poor
outcomes, including patient demographics (anatomy, prematurity, weight,
genetic syndrome), biological markers, and the creation of complexity
scores [6-8]. However, there is a need to identify and quantify clinical
factors during the early postoperative period that are indicative of
short-term outcomes including mortality.
The repair or palliation of CHD often results in a
decrease in cardiac output during the immediate postoperative period,
and inotropic and vasoactive agents are routinely used after cardiac
surgery in infants [9]. The Aristotle Basic Complexity (ABC) score in
CHD surgery is a consensus-based scoring system developed early in 2000s
in order to provide risk adjustment, and thus allow for a standardized
comparison of performance between institutions [10-12]. In 1995,
Wernovsky, et al. [13] created an Inotrope score [IS] aimed to
quantify the amount of inotropic support provided in the postoperative
period, which is frequently used for outcomes. More recently, Gaies,
et al. [14] developed and tested vasoactive-inotropic score (VIS) in
children <6 months of age undergoing cardiac surgery with
cardiopulmonary bypass (CPB). VIS encompasses the original medications
from the IS and adds milrinone, vasopressin, and norepinephrine.
However, there is little information on the predictive values of VIS for
mortality in newborns with CHD [15,16]. Therefore, we aimed to assess
the predictive values of VIS for mortality and to compare them with ABC
score in this population. We hypothesized that maximum VIS in the first
72 hours after cardiac surgery might predict mortality, and that we
could define a cut-point that would effectively discriminate patients
likely to have mortality in the postoperative period.
Methods
Our Neonatal intensive care unit (NICU) – Congenital
heart disease (CHD) center serves as a reference hospital for newborns
with CHD. In our NICU, the patients with CHD are followed-up in
consultation with pediatric cardiologists. Cardiac surgeries can be
performed in two centers (Dr Sami Ulus Training and Research Hospital,
Ankara and Turkiye Yuksek Ihtisas Training and Research Hospital,
Ankara). Sometimes cardiovascular interventions may be performed late
because of wrong/late diagnosis of CHD in another center, delayed
transfer of the patients, under-staffing of the departments, and
clinical condition of the patients.
This was a prospective study enrolling consecutive
newborns with CHD who were admitted to the NICU between November 2016
and January 2019. The newborns who did not require an urgent surgery, or
those who died before the surgery were excluded. Informed written
consent were obtained from the parents of all participants.
Basic demographic and clinical information of the
patients including gender, gestational age and birthweight at delivery,
postnatal age at surgery, anatomic diagnosis and surgical procedure were
extracted from the patients’ files and/or hospital computer-based
system. Post-operative variables included duration of mechanical
ventilation, presence of sepsis, pneumonia, hemato-logical dysfunction,
liver dysfunction, renal failure (requiring dialysis), LCOS (oliguria,
tachycardia, poor perfusion, or cardiac arrest that required high dose
inotropic support), multiorgan dysfunction syndrome (MODS) ( ł2
organ dysfunctions), NICU length of stay, and mortality. Mortality was
defined as the patient dying after surgery but before discharge from
hospital, or death after hospital discharge but within 30 postoperative
days.
All data were recorded in pre-tested structured
forms. SNAPPE-II scores that predicts neonatal mortality on admission
were noted for each patient [9,17,18]. For evaluating the cardiac
surgery-related mortality risk, operations were categorized by using the
ABC [11]. Scoring in ABC system varies between 1.5 and 15 and there are
4 difficulty levels (1.5-5.9=1st level, 6.0-7.9=2nd level, 8.0-9.9=3rd
level, 10.0-15.0=4th level).
According to our unit protocol, inotropic and
vasoactive medications were initiated in the operating room at the
discretion of the attending surgeon and pediatric cardiologist.
Decisions regarding ongoing titration of vasoactive/inotropic
medications were made by the NICU physician team based on each patient’s
physiological state and did follow our NICU-CHD guidelines.
Considerations involved in the choice of medications included
ventricular function, echo-cardiographic findings, and physiological
parameters. The patients received milrinone and dopamine/dobutamine as
first-line inotropic agents. The second-line agents were often
epinephrine for hypotension with ventricular dysfunction or vasopressin
or terlipressin for hypotension without ventricular dysfunction.
For each patient, VIS values were calculated by a
standard formula for the first 72 postoperative hours, and the maximum
score was recorded [14]. VIS: Dopamine dose (µg/kg/min) + dobutamine
dose (µg/kg/min) + 100 × epinephrine dose (µg/kg/min)] + 10 × milrinone
dose (µg/kg/min) + 10,000 × vasopressin dose (Units/kg/min) + 100 ×
norepinephrine dose (µg/kg/min).
Primary outcome was duration of mechanical
ventilation. Secondary outcomes were NICU length of stay and mortality
at 30 days postoperatively.
Statistical analyses: Statistical analyses were
performed with IBM-SPSS (version 25, Chicago, SPSS Inc.), and P<0.05
was considered significant. Normality of data was analyzed by using
Kolmogorov-Smirnov test. Demographic and clinical characteristics were
compared between groups using Chi-square test for categorical variables
and t-test or Mann Whitney U test, as appropriate, for continuous
variables. Spearman correlation test was used for correlations.
To determine the predictive values for mortality,
Area under the curve (AUC) of VIS was defined and compared with ABC.
The best cut-off was chosen utilizing sensitivity and specificity from
the Receiver operating characteristic (ROC) curve of the selected data.
For multiple logistic regression modeling, variables that possibly
related to mortality in newborns with CHD (gestational age, birthweight,
5 min APGAR score, age at diagnosis, age at surgery, SNAPPE-II, ABC and
VIS) were chosen as candidate covariates.
Results
During the study period a total of 144 newborns with
CHD were admitted to our NICU-CHD center. Among them 25 were not
included in the study; 18 discharged without surgery (therapeutic
catheterization or clinical follow-up), 7 died preoperatively. Finally
119 newborns who underwent cardiac surgery and followed up in our NICU
postoperatively were included in the study.
Of 119 patients (53.3% males), the mean (SD)
gestational age and weight were 38.3 (1.6) week and 3110 (550) g,
respectively. The median (IQR) ages at diagnosis of CHD and NICU
admission were 1.5 (1-5) and 3 (2-9) days. The rate of prenatal
diagnosis was 31 (26%). None of the patient was diagnosed by pulse
oximeter screening test for CHD. About 79.8% (n=95) of the
patients were transferred to our unit from another NICU center. The most
common presentation findings for CHD were respiratory distress (28.3%)
and cyanosis (28.3%). Left sided (n=47, 39.5%), right sided (n=27,
22.7%), and mixing lesions (n=36, 30.3%) were the commonest (Table
I).
TABLE I Type of Congenital Heart Diseases (CHD) in Neonates Enrolled in the Study (N=119)
|
Type of CHD |
No. (%) |
|
Left sided lesions |
47 (39.5)
|
|
Coarctation of aorta/left ventricular hypoplasia |
33 (27.7) |
|
Hypoplastic left heart syndrome
|
8 (6.7) |
|
Aortic interruption |
6 (5.0) |
|
Right sided lesions |
27 (22.7) |
|
Pulmonary stenosis/atresia
|
20 (16.8) |
|
Double outlet right ventricle
|
5 (4.2) |
|
Hypoplastic right heart syndrome |
1 (0.8) |
|
Tetralogy of Fallot |
1 (0.8) |
|
Mixing pathologies |
36 (30.3) |
|
Transposition of great arteries
|
28 (23.5) |
|
TAPVC |
3 (2.5) |
|
Truncus arteriosus |
4 (3.4) |
|
Univentricular heart disease |
1 (0.8) |
|
Others |
9 (7.6) |
|
Atrioventricular septal defect |
4 (3.4) |
|
PDA |
5 (4.2) |
|
CHD: Congenital heart disease, PDA: Patent ductus arteriosus,
TAPVC: Total anomalous pulmonary venous connection. |
At surgery, the median (IQR) age was 15 days (9-31).
Overall mortality rate was 30.3%. The patients were divided into two
groups according to mortality; Group 1 (Non-survivors) (n=36) and
Group 2 (Survivors) (n=83). Table II shows
demographic and clinical details of all patients by groups. Gestational
age, birthweight, and gender were similar in groups. Duration of
mechanical ventilation was longer in Group 1 (P<0.001);
postoperative complications were more frequent among non-survivors (P<0.001).
NICU length of stay was shorter in non-survivors (P=0.03); VIS
score was significantly higher in Group 1 compared to Group 2 (P=0.003).
TABLE II Demographic and Clinical Characteristics of the Patients by Mortality Groups
|
Non-survivors (n=36) |
Survivors (n=83) |
P value |
|
Gestational age (wk) |
37.9 (2.1) |
38.4 (1.2) |
0.18 |
|
Gender (male), n (%) |
10 (52.6) |
22 (53.7) |
0.94 |
|
Birthweight (g) |
2986 (624) |
3167 (514) |
0.24 |
|
*APGAR, 5 min |
9 (8-9) |
9 (7-9) |
0.87 |
|
*Admission age (d) |
2 (1-9) |
4 (2-10) |
0.21 |
|
*Age at diagnosis (d) |
1 (1-3) |
2 (1-7) |
0.09 |
|
*Age at surgery (d) |
14 (8-28) |
17 (9-33) |
0.07 |
|
CHD type, n% |
|
Left sided |
14 (38.9) |
33 (39.8) |
|
|
Right sided |
8 (22.2) |
19 (22.9) |
|
|
Mixing |
13 (36.1) |
23 (27.7) |
|
|
Other |
1 (2.8) |
8 (9.6) |
0.82 |
|
Operation type, n% |
|
COA. resection/Hypoplastic arch repair |
8 (22.2) |
25 (30.1) |
|
|
BT shunt
|
6 (16.7) |
20 (24) |
|
|
Pulmonary banding
|
1 (2.8) |
8 (9.6) |
|
|
Arterial switch
|
10 (28) |
18 (21.7) |
|
|
Pulmonary valvulasty
|
1 (2.8) |
1 (1.2) |
|
|
Hybrid procedure
|
4 (11.1) |
- |
|
|
Truncal repair
|
3 (8.3) |
1 (1.2) |
|
|
Norwood |
3 (8.3) |
- |
|
|
TAPVC |
- |
3 (3.6) |
|
|
PDA ligation |
- |
5 (6.0) |
0.04 |
|
APW repair |
- |
1 (1.2) |
|
|
ASD/VSD repair |
- |
1 (1.2) |
|
|
*Duration of mechanical ventilation (d) |
15 (8-23) |
7 (4-11) |
<0.001 |
|
Postoperative complications, n(%) |
|
LCOS/Arrythmia |
10 (27.7) |
7 (8.4) |
|
|
Sepsis/Pneumonia |
4 (11.1) |
9 (10.8) |
|
|
Renal failure (dialysis) |
4 (11.1) |
3 (3.6) |
|
|
Necrotizing enterocolitis |
3 (8.3) |
- |
|
|
Pulmonary hemorrhage |
2 (5.5) |
1 (1.2) |
|
|
MODS |
5 (13.8) |
1 (1.2) |
<0.001 |
|
Diaphragm paralysis |
- |
4 (4.8) |
|
|
SNAPPE-II
|
20.8 (16.1) |
15.9 (12) |
0.19 |
|
Aristotle basic complexity score (ABC) |
7.6 (2.3) |
7.1 (1.7) |
0.37 |
|
Vasoactive inotropic score (VIS) |
49.8 (45.4) |
13.9 (18.5) |
0.003 |
|
NICU length of stay (d) |
22 (15)
|
36 (28) |
0.03 |
|
ASD/VSD: Atrial/ventricular septal defect, APW:
Aorticopulmonary window, CHD: Congenital heart disease, LCOS:
Low cardiac output syndrome, MODS: Multiorgan dysfunction
syndrome, NICU: Neonatal intensive care unit, PDA: Patent ductus
arteriosus, SNAPPE-II: Score for Neonatal Acute Physiology with
Perinatal Extension-II, TAPVC: Total anomalous pulmonary venous
connection, All variables are expressed as mean (SD) except
*median (IQR). |
Greater VIS score was correlated to longer duration
of mechanical ventilation (P=0.009, r=0.33). No
correlation was detected with VIS, ABC, and NICU length of stay (P
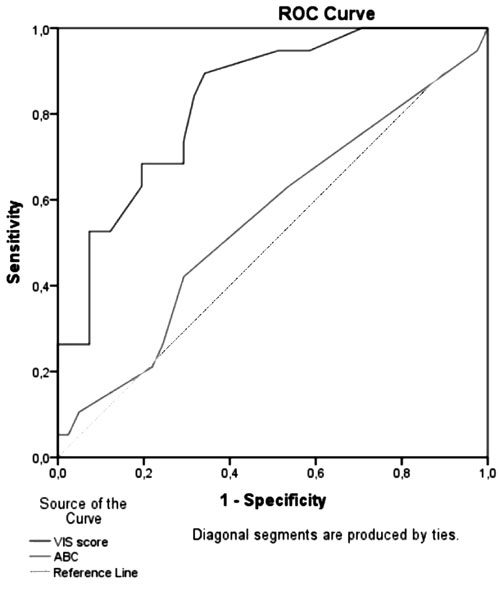
>0.05). As seen in Fig. 1, VIS was the more successful in
predicting deaths compared to ABC. Area under the curve (AUC) was 0.83 (P
<0.001, 95% CI: 0.7-0.9) for VIS to identify mortality. AUC was 0.55
for ABC (P = 0.52). At a cut-off value of 15.5, as defined by ROC
analysis, sensitivity, specificity, the positive and negative predictive
values of VIS for mortality were 73.6%, 70.7%, 53.8%, and 85.3%.
 |
|
Fig. 1 Receiver operating
characteristic (ROC) curve for Aristotle basic complexity (ABC)
score, and vasoactive-inotropic score (VIS) for prediction of
<30-day mortality after cardiac surgery.
|
When gestational age, birtweight, 5 min APGAR score,
age at diagnosis, age at surgery, SNAPPE-II, ABC and VIS were entered in
the model, multivariate analysis showed that higher VIS (>15.5) was
independently associated with increased odds for mortality (OR: 8.1, 95%
CI: 1.8-35.7, P = 0.005).
Discussion
In this study, we compared ABC and VIS scores in
newborns after cardiac surgery. Our findings confirm that VIS, assessed
in the first 72 hours after surgery, predicts mortality better than ABC
in this population.
The ABC score, a procedure-adjusted scoring system,
contains the sum of the potential for mortality, morbidity, and the
anticipated surgical difficulty of the procedures. ABC is an appropriate
tool for evaluating many CHD procedures. O’Brien, et al. [19]
reported that when ‘prolonged hospital stay’ (>21 days) was chosen as a
marker of morbidity, there was a significant positive correlation with
the ABC score. Similarly, Erek, et al. [20] found that a longer
ICU stay showed a significant correlation with ABC scores. In the
current study, maximum score for ABC was 14.5 in non-survivors and 11 in
survivors, without significantly. We could not find any association
between ABC and length of NICU stay or mortality. This may be caused by
early death of more severely ill patients in our population. In
non-survivors, although they were diagnosed at 2 day of life, surgery
was not undertaken until average 14 days because of sepsis, hemodynamic
instability or inadequate equipment.
Kumar, et al. [21] performed a retrospective
analysis of 208 patients who underwent cardiac surgery for CHD and
reported that VIS was an excellent tool to measure illness severity,
deciding interventions, and during parental counseling in the pediatric
cardiac surgery ICUs.
Sanil and Aggarwal [22] computed peak VIS within the
initial 24 and 48 h after 51 consecutive open heart transplants. They
have observed that the patients with peak VIS
ł15 constituted the
high VIS group and these patients had significantly longer ICU stay,
inotropic requirement and ventilatory durations, and higher rates of
short-term morbidities. Recently, Yamazaki, et al. [23] reported
that amount of cardiovascular support with high VIS at the end of
surgery might predict morbidity and mortality in adults. Gaies, et al.
[14] conducted a study among 391 infants with 141 (36%) neonates and
determined empirically high VIS to be maximum VIS >15 in the first 24 h.
They reported high VIS was significantly associated with 30-day
mortality, duration of mechanical ventilation and ICU stay. In our
study, VIS scores were significantly higher among deceased patients;
maximum score was 140 in non-survivors and 75 in survivors,
respectively.
Davidson, et al. [16] reported that higher VIS
at 48 hours after cardiothoracic surgery was strongly associated with
increased length of ventilation, prolonged ICU and total hospital stay
in neonates and infants. Similarly, we found a strong association
between VIS and duration of mechanical ventilation, but not NICU stay.
During the postoperative period, newborns generally require high levels
of inotropic and vasoactive support, and clinicians were less likely to
extubate the patient. It is likely that therapies capable of improving
postoperative VIS would directly improve intubation times as well. The
relationship between high VIS and prolonged NICU stay is also an
important issue. In many cases, NICU length of stay prolonged due to
factors not directly associated with VIS such as sepsis, pneumoniae,
MODS, poor feeding, phrenic nerve injury, and chylothorax. It is
possible that high VIS is simply a marker for poor physiology in the
early postoperative period. However, this poor physiology may later lead
to prolonged therapies, feeding intolerance, impaired cardiac and
pulmonary functions. In this study, no correlation between VIS and NICU
length of stay can be explained with the earlier death of the patients
with higher VIS.
Our study has a number of limitations. It reflects a
single NICU experience in a well defined neonatal population. The
performance of the surgeries in two different centers might have affect
the postoperative outcome of the patients. Although the clinical
management was under protocol, patient progression may have been
affected by variations in the practices of neonatologist in charge.
However, it is an important study that defines predictive values of VIS
in newborns with CHD after cardiac surgery by comparing them with ABC.
The Vasoactive-Ventilation-Renal (VVR) score is a
novel disease severity index that incorporates validated markers of
cardiovascular, pulmonary, and renal function [24]. Recently, in a
multicenter cohort study performed on newborns who underwent cardiac
surgery, Cashen, et al. [24] showed that the VVR was a reliable
predictor of postoperative outcome and outperformed more traditional
measures of disease complexity and severity. Another score available is
Society of Thoracic Surgeons - European Association for Cardio-Thoracic
Surgery Congenital Heart Surgery Mortality Categories (STAT Mortality
Category) [25] for prediction of morbidity and mortality risk in
newborns underwent CHD surgery.
The results of this study showd that VIS is more
useful than ABC in prediction of neonatal mortality in newborns after
cardiac surgery. A high VIS (>15.5) within 72 hours should trigger
clinician awareness that the infant in question continues to be at risk
for poor outcome. Further research should be designed as multi-centered
and adequately powered to detect small differences in short term
outcomes, especially mortality. Long term follow-up is needed to
evaluate neurologic outcome and long term morbidity and mortality.
Contributors: DD, IT: concept and designed the
study, analyzed data and drafted the manuscript; UAO, MM, RÇ, SA, HA,
ST: collected the data and helped in data analysis; IT, DD: supervised
final manuscript.
Funding: None; Competing Interest: None
stated.
|
What is Already Known?
• There is little data on the predictive
ability of Vasoactive-inotrope Score (VIS) for mortality in
newborns with congenital heart disease (CHD).
What This Study Adds?
• In newborns with CHD undergoing surgery, VIS measured in
early postoperative period (<72 hours), may be useful for
prediction of mortality.
|
References
1. Cho SY, Oh JH, Lee JH, Lee JY, Lee SJ, Han JW,
et al. Recent incidence of congenital heart disease in neonatal care
unit of secondary medical center: A single center study. Korean J
Pediatr. 2012; 55:232-7.
2. Başpinar O, Karaaslan S, Oran B, Baysal T, Elmaci
AM, Yorulmaz A. Prevalence and distribution of children with congenital
heart diseases in the central Anatolian region, Turkey. Turk J Pediatr.
2006: 48:237-43.
3. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE,
Correa A. Mortality resulting from congenital heart disease among
children and adults in the United States, 1999 to 2006. Circulation.
2010;122:2254-63.
4. Saxena A. Congenital heart disease in India: A
status report. Indian Pediatr. 2018;55:1075-82.
5. Tchervenkov CI, Jacobs JP, Bernier PL, Stellin G.
The improvement of care for paediatric and congenital cardiac disease
across the World: A challenge for the World Society for Pediatric and
Congenital Heart Surgery. Cardiol Young. 2008;18:63-9.
6. Dorfman AT, Marino BS, Wernovsky G, Tabbutt S,
Ravishankar C, Godinez RI, et al. Critical heart disease in the
neonate: Presentation and outcome at a tertiary care center. Pediatr
Crit Care Med. 2008;9:193-202.
7. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL,
Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for
surgery for congenital heart disease. J Thorac Cardiovasc Surg.
2002;123:110-8.
8. Seear MD, Scarfe JC, LeBlanc JG. Predicting major
adverse events after cardiac surgery in children. Pediatr Crit Care Med.
2008; 9:606-11.
9. Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson
DP, Chang AC, et al. Efficacy and safety of milrinone in
preventing low cardiac output syndrome in infants and children after
corrective surgery for congenital heart disease. Circulation.
2003;107:996-1002.
10. Richardson DK, Corcoran JD, Escobar GJ, Lee M SK.
SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality
risk scores. J Pediatr. 2001;138: 92-100.
11. Lacour-Gayet F, Clarke D, Jacobs J, Comas J, Daebritz
S, Daenen W, et al. The Aristotle score: a complexity-adjusted
method to evaluate surgical results. Eur J Cardiothorac Surg.
2004;25:911-24.
12. Joshi SS, Anthony G, Manasa D, Ashwini T,
Jagadeesh AM, Borde DP, et al. Predicting mortality after
congenital heart surgeries: Evaluation of the aristotle and risk
adjustement in congenital heart surgery-1 risk prediction scoring
systems: A retrospective single center analysis of 1150 patients. Ann
Card Anaesth. 2014;17:266-70.
13. Wernovsky G, Wypij D, Jonas RA, Mayer JEJ, Hanley
FL, Hickey PR, et al. Postoperative course and hemodynamic
profile after the arterial switch operation in neonates and infants. A
comparison of low-flow cardiopulmonary bypass and circula-tory arrest.
Circulation. 1995; 92:2226-35.
14. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski
RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of
morbidity and mortality in infants after cardiopulmonary bypass. Pediatr
Crit Care Med. 2010;11:234-8.
15. Butts RJ, Scheurer MA, Atz AM, Zyblewski
SC, Hulsey TC, Bradley SM, et al. Comparison of maximum
vasoactive inotropic score and low cardiac output syndrome as markers of
early postoperative outcomes after neonatal cardiac surgery. Pediatr
Cardiol. 2012;33:633-8.
16. Davidson J, Tong S, Hancock H, Hauck A, da Cruz
E, Kaufman J. Prospective validation of the vasoactive-inotropic score
and correlation to short-term outcomes in neonates and infants after
cardiothoracic surgery. Intensive Care Med. 2012;38:1184-90.
17. Reid S, Bajuk B, Lui K, Sullivan EA; NSW and ACT
Neonatal Intensive Care Units Audit Group, PSN. Comparing
CRIB-II and SNAPPE–II as mortality pre-dictors for very preterm infants.
J Paediatr Child Health. 2015;51:524-8.
18. Jain S, Bansal A. SNAPPE II score for predicting
mortality in a level II neonatal intensive care Unit. Dicle Med J.
2009;36:333-5.
19. O’Brien SM, Jacobs JP, Clarke DR, Maruszewski
B, Jacobs ML, Walters HL 3rd, et al. Accuracy of the aristotle
basic complexity score for classifying the mortality and morbidity
potential of congenital heart surgery operations. Ann Thorac Surg.
2007;84:2027-37.
20. Erek E, Yýlmaz B, Kaya M, Onan ÝS, Ţen O, Öz K.
et al. Analysis of results according to the Aristotle scoring
system in congenital heart surgery. Turkish J Thoracic Cardiovascular
Surgery. 2014;22:509-16.
21. Kumar M, Sharma R, Sethi SK, Bazaz S, Sharma P, Bhan
A, et al. Vasoactive inotrope score as a tool for clinical care
in children post cardiac surgery. Indian J Crit Care Med. 2014;18:653-8.
22. Sanil Y, Aggarwal S. Vasoactive-inotropic score
after pediatric heart transplant: A marker of adverse outcome. Pediatr
Transplant. 2013;17:567-72.
23. Yamazaki Y, Oba K, Matsui Y, Morimoto Y.
Vasoactive –inotropic score as a predictor of morbidity and mortality in
adults after cardiac surgery with cardiopulmonary bypass. J Anesth.
2018;32:167-73.
24. Cashen K, Costello JM, Grimaldi LM, Narayana
Gowda KM, Moser EAS, Piggott KD, et al. Multicenter validation of
the vasoactiveventilation-renal score as a predictor of prolonged
mechanical ventilation after neonatal cardiac surgery. Pediatr Crit Care
Med. 2018;19:1015-23.
25. Jacobs JP, Mayer JE Jr, Pasquali SK, Hill KD,
Overman DM, St Louis JD, et al. The Society of Thoracic Surgeons
Congenital Heart Surgery Database: 2018 Update on Outcomes and Quality.
Ann Thorac Surg. 2018;105: 680-9.
|
|
|
 |
|

