|
|
|
Indian Pediatr 2015;52:
769-772 |
 |
Pulse Oximetry Screening
to Detect Cyanotic Congenital Heart Disease in Sick Neonates in
a Neonatal Intensive Care Unit
|
|
NB Mathur, A Gupta and *S
Kurien
From the Department of Neonatology, Maulana Azad
Medical College; and *Department of Cardiology, GB Pant Hospital;
New Delhi, India.
Correspondence to: Dr NB Mathur, Director-Professor
and Head, Department of Neonatology, Maulana Azad Medical College,
New Delhi, India.
Email: drnbmathur@gmail.com
Received: November 29, 2014;
Initial review: January 01, 2015;
Accepted: July 01, 2015.
|
Objective: To evaluate pulse oximetry for detection of congenital
cyanotic heart disease in sick neonates using echocardiography as gold
standard.
Methods: Pulse oximetry readings were taken at
admission from 950 neonates from right upper limb and either foot with
infant breathing room air. Pulse oximetry was considered abnormal if
oxygen saturation at room air measured <90% or difference between right
hand and foot was more than 3%. Persistent abnormality was considered
positive result. Echocardiography was performed on all neonates with
positive pulse oximetry (study group) and on one subsequent neonate with
negative screen for each neonate with positive screen (controls).
Results: Pulse oximetry was positive in 210
neonates. It detected 20 out of 21 (95.2%) true positives. The
sensitivity, specificity, positive predictive value, negative predictive
value and odds ratio (95% CI) of pulse oximetry was 95.2%, 52.4 %, 9.5,
99.5 and 22 (5.3, 91.4), respectively.
Conclusion: Pulse oximetry screening is useful in
detecting cyanotic heart diseases in sick newborns.
Keywords: Cyanosis, Duct-dependent lesions, Oxygen saturation,
Screening.
|
|
C
ongenital heart diseases (CHDs) account for 6 -10
% of all the infant deaths, and 20 - 40 % of all infant deaths from
malformations [1]. About 25%
of CHDs are life threatening and manifest before the first routine
clinical examination [1,2]. Challenges in managing CHD in developing
countries include delay in diagnosis, transport of sick neonate to
tertiary centre, and limited availability of state of the art pediatric
cardiac centres [3,4].
The existing pulse oximetry monitoring protocol to
detect critical congenital heart disease, is restricted to neonates 24
to 48 hours of age in well infant nursery [5]. A simple algorithm for
units catering to sick newborns is challenging because of heterogeneity
of underlying conditions; need of studies across a broad range of
newborn delivery systems has been expressed [5]. Pulse oximetry as a
screening test for congenital cyanotic heart disease has been evaluated
among well neonates [2,6-10], but not in sick neonates.The present study
was designed to evaluate the utility of pulse oximetry screening in
detecting congenital cyanotic heart disease among sick neonates in a
referral neonatal unit catering to outborn neonates.
Methods
The study was conducted in the Referral Neonatal Unit
of a teaching hospital between April 2013 and January 2014. The unit
caters exclusively to outborn sick neonates referred from community
hospitals of Delhi and surrounding states, or to those born at home and
transported to the hospital directly by the parents. All neonates
admitted to the unit during the study period were eligible for
inclusion. Neonates in whom stable pulse oximeter signals could not be
obtained were excluded. Informed written consent was obtained from the
parents of all enrolled subjects. The study was approved by the
Institutional ethical committee.
All neonates at admission were clinically evaluated
by a resident doctor for temperature, heart rate, respiratory rate (RR),
chest retractions, central cyanosis, femoral pulses, other peripheral
pulses, capillary filling time, peripheries (cool or warm) and clubbing.
Presence of either tachypnea (RR >60/min), retractions, central
cyanosis, poor femoral pulses, precordial pulsations, hepatomegaly or
murmur was considered as positive clinical examination suggestive of
congenital heart diease [11]. Pulse oximetry readings (BPL Excello
oximeter with reusable Nellcor Oximax probe accuracy of + 2 %)
were taken at admission from right upper limb and either foot with
infant breathing room air. The recordings were noted two minutes after
stable signals were obtained. Pulse oximetry was considered abnormal if
oxygen saturation at room air or on oxygen therapy measured <90% or
there was more than 3% difference between right hand and foot [5]. All
neonates with abnormal pulse oximetry were subjected to three
observations each, separated by at least 1 hour. Screen was considered
positive only if the abnormality persisted till the last reading.
Echocardio-graphy (Philips iE33 xMATRIX echocardiography system) was
performed by a pediatric cardiologist on all neonates with a positive
pulse oximetry screen (study group) and on one subsequently enrolled
neonate with negative screen per neonate with positive screen
(controls).
Data were analyzed using Statistical Package for
Social Sciences software (version 21). Student-t test was used for
continuous variables and Chi-square test was used for comparing
proportions. Multivariate logistical regression (using the forward
logistical regression model) was done to find predictors of cyanotic
heart disease. Sensitivity, specificity, positive and negative
predictive value, positive likelihood ratio and negative likelihood
ratio of pulse oximetry in detecting cyanotic heart disease in sick
neonates were calculated.
Results
A total of 950 neonates admitted in referral neonatal
unit during the study period were screened. Pulse oximetry was positive
in 210 neonates, and in five neonates, stable pulse oximeter signals
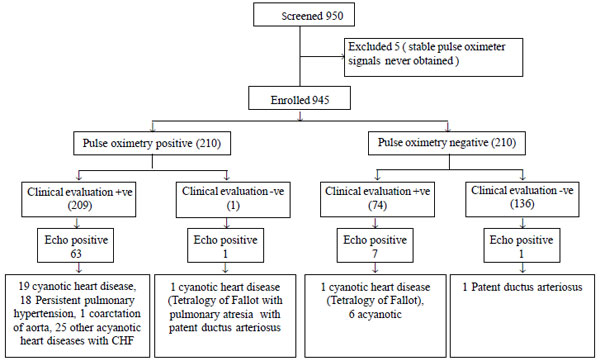
could not be obtained. Fig. 1 shows flow of the study. Out
of the 210 controls, 11 neonates (3 with shock, 3 with transient
tachypnea of newborn and 5 with pneumonia) had an initial saturation of
<90%, but repeat readings were >90%.
 |
|
Fig. 1 Flow of participants in the
study.
|
Table I compares the baseline demographic and
clinical characteristics of cases and controls. Pulse oximetry was
positive in 20 out of 21 (95.2%) neonates with echocardiography proven
cyanotic heart disease. This included lesions with increased pulmonary
blood flow (d-transposition of great arteries, n=8), lesions with
decreased pulmonary blood flow (tetrology of Fallot, n=5; double
outlet right ventricle with pulmonary atresia/severe pulmonary stenosis,
n=3; single ventricle with pulmonary stenosis, n=1) and
lesions with pulmonary venous hypertension (obstructed total anomalous
pulmonary venous connection, n=3). Pulse oximetry was negative in
1 neonate with tetralogy of Fallot with mild pulmonary stenosis and
large left to right shunt.
TABLE I Comparison of Characteristics of Cases and Controls
|
Characteristic |
Cases |
Controls |
|
(n=210) |
(n=210) |
|
Age (h), median (IQR) |
72 (24, 183) |
120 (48, 240) |
|
Male, No. (%) |
137 (65.2) |
125 (59.4) |
|
Gestational age (wk), mean (SD) |
37.7 (2.2) |
37.4 (2.5) |
|
#Weight (g), median(IQR) |
2500 (2081,2900) |
2335 (1800, 2786) |
|
Family h/o smoking, No.(%) |
9 (4.3) |
12 (5.7) |
|
Clinical signs, No. (%) |
|
Tachycardia >160/min |
33 (15.7) |
6 (2.9) |
|
Tachypnea >60/min |
143 (68.1) |
25 (11.9) |
|
Chest retractions |
114 (54.3) |
69 (32.9) |
|
Central cyanosis |
46 (21.9) |
0 |
|
Murmur |
47 (22.4) |
10 (4.8) |
|
Feeble femoral pulses |
32 (15.2) |
9 (4.3) |
|
Hepatomegaly |
37 (17.6) |
14 (6.7) |
|
Disease categories |
|
Cyanotic heart disease |
20 ( 9.5) |
1 (0.5) |
|
Acyanotic heart disease |
26 (12.4) |
7 (3.3) |
|
Persistent pulmonary hypertension |
18 (8.6) |
0 |
|
Respiratory diseases |
115 ( 54.8) |
24 (11.4) |
|
Shock |
31 ( 14.8) |
9 (4.3) |
|
Others |
0 |
169* (80.4 ) |
|
*Sepsis 89, hypoxic ischemic encephalopathy 30, hyper-bilirubinemia
29, healthy preterms 17, hemorrhagic disease of newborn 4;
#Admission weight. |
The sensitivity, specificity, positive predictive
value, negative predictive value, positive likelihood ratio, negative
likelihood ratio and odds ratio (95% CI) of pulse oximetry to detect
cyanotic congenital heart disease was 95.2%, 52.4% 9.5%, 99.5%, 2.0, 0.1
and 22 (5.3, 91.4), respectively . The sensitivity of pulse oximetry to
detect critical congenital heart disease (cyanotic heart disease, n=21
and critical duct dependent systemic lesion, n=1) and PPHN (n=18)
was 97.5 % (39/40) with negative predictive value of 99.5% (209/210).
Table II shows pulse oximetry findings in
study population. The pre-post ductal difference was >3% only in cases
of cyanotic heart disease, coarctation of aorta and persistent pulmonary
hypertension of newborn.
TABLE II Pulse Oximetry Findings in Study Population
|
SpO2 <90 % |
Pre-post ductal difference >3% |
SpO2
90-<95% |
SpO2
≥95% |
|
Cyanotic heart disease (n=21) |
20 (95.2%) |
5 (23.8%) |
1 (4.8%) |
0 |
|
Acyanotic heart disease (n=33) |
26 (78.8%) |
1 (3.0%) |
4 (12.1%) |
3 (9.1%) |
|
Persistent pulmonary hypertension (n=18) |
18 (100%) |
8 (44.4%) |
0 |
0 |
|
Respiratory diseases (n=139) |
115 (82.7%) |
0 |
13 (9.4%) |
11 (7.9%) |
|
Shock (n=40) |
31 (77.5%) |
0 |
8 (20%) |
1 (2.5 %) |
|
Others (n=169) |
0 |
0 |
95 (56.2%) |
74 (43.8%) |
On univariate analysis significant predictors of
cyanotic heart disease among sick neonates was positive pulse oximetry,
male gender, history of consanguinity, history of pregnancy induced
hypertension in mother, family history of congenital heart disease and
smoking, and presence of tachycardia, central cyanosis, murmur or
hepatomegaly. The significant predictors of cyanotic heart disease among
sick neonates on multivariate analysis are outlined in Table
III.
TABLE III Predictors of Cyanotic Heart Disease in Sick Neonates
|
Predictor |
P value |
OR (95% CI) |
|
Positive pulse oximetry screen |
< 0.001 |
12.9 (3.4, 49.9) |
|
Male gender |
0.023 |
89.3 (1.9,4194.1) |
|
Consanguinity |
0.003 |
282.3 (6.6,11254.9) |
|
PIH |
0.003 |
62.8 (4.2, 94.1) |
|
Family history of smoking |
0.011 |
45.5 (2.4,858.5) |
|
Central cyanosis |
<0.001 |
653.3 (30.5, 13998.8) |
|
Murmur |
<0.001 |
962.8 (30.0, 30889.1) |
|
PIH – Pregnancy induced hypertension. |
Discussion
In the present study, all congenital heart diseases
with different hemodynamics, except one case of tetralogy of Fallot,
were detected using pulse oximetry screen. The sensitivity and negative
predictive values of pulse oximetry screening to detect cyanotic heart
disease and critical congenital heart disease were high. Murmur, central
cyanosis, male gender, consanguinity, family history of smoking and
history of pregnancy induced hypertension were significant predictors of
cyanotic heart disease.
Majority of the studies done in well infant nurseries
had used the saturation cut-off of less than 95% for abnormal pulse
oximetry [7,9,10]. The
working group [5] recommended any saturation below 90% as abnormal for
pulse oximetry screening in well infant nursery, and recommended three
repeated saturations taken hourly if the saturation is between 90% and
95%. In our study, only one case of cyanotic heart disease (teralogy of
Fallot) had a saturation persisting between 90% and 95% . Considering a
persistent saturation value of <95% as criteria for positive pulse
oximetry screen would have led to 120 additional referrals for
echocardiography. Specificity of pulse oximetry was low because it was
also positive in cases of respiratory diseases, acyanotic heart diseases
with congestive heart failure, shock and persistent pulmonary
hypertension which are common in neonatal intensive care settings.
Persistent pulmonary hypertension and hypoxic cardiac conditions have
been considered as secondary targets of pulse oximetry screening
[5,9,12].
The limitations of present study include single
center-based enrolment, and no a priori sample size calculation.
Also, echocardiography was done only on selected controls rendering
calculations of sensitivity, specificity and predictive values
inaccurate.
To conclude, pulse oximetry screening is useful in
detecting cyanotic heart diseases in a setting catering to sick out born
neonates. Negative predictive value of pulse oximetry is high, making it
useful to reliably rule out critical congenital heart disease or PPHN
among sick neonates, thus avoiding need for an urgent echocardiography.
|
What This Study Adds?
• Pulse oximetry screening is useful in detecting cyanotic
heart diseases, critical duct-dependent systemic lesions and
persistent pulmonary hypertension in sick neonates.
|
References
1. Wren C, Reinhardt Z, Khwaja K. Twenty year trends
in diagnosis of life threatening neonatal cardiovascular malformations.
Arch Dis Child Fetal Neonatal Ed. 2008;93:F33-7.
2. Vaidyanathan B, Satish G, Mohannan ST, Sundaram
KR, Warrier KKR, Kumar RK. Clinical screening for congenital heart
disease at birth: A prospective study in a community hospital in Kerala.
Indian Pediatr. 2011;48:25-30.
3. Bakshi KD, Vaidyanathan B, Sundaram KR, Roth SJ,
Shivaprakasha K, Rao SG, et al. Determinants of early outcome
after neonatal heart surgery in a developing country. J Thoracic Cardio
Vascular Surgery. 2007;134:765-71.
4. Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci
M, Bull C. Delayed diagnosis of congenital heart disease worsens
preoperative condition and outcome of surgery in neonates. Heart.
2006;92:1298-302.
5. Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar
P, Morrow WR, et al. Strategies for implementing screening for
critical congenital heart disease. Pediatrics. 2011;128:e1259-67.
6. Sendelbach DM, Jackson GL, Lai SS, Fixler DE,
Stehel EK, Engle WD. Pulse oximetry screening at 4 hours of age to
detect critical congenital heart defects. Pediatrics. 2008;122:e815-20.
7. Ewer AK, Middleton LJ, Furmston AT, Bhoyar
A, Daniels JP, Thangaratinam S, et al. Pulseox Study Group. Pulse
oximetry screening for congenital heart defects in newborn infants (Pulseox):
A test accuracy study. Lancet. 2011;378:785-94.
8. Arlettaz R, Bauschatz AS, Monkoff Messers B,
Bauersfeld U. The contribution of pulse oximetry for early diagnosis of
congenital heart disease in newborns. Eur J Pediatr. 2006;165:94-8.
9. de-Wahl Granelli A, Wennergren M, Sandberg K,
Mellander M, Bejlum C, Inganas N, et al. Impact of pulse oximetry
screening on the detection of duct dependent congenital heart disease: A
Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:
a3037.
10. Meberg A, Andreasson A, Brunvand L, Markestad T,
Moster D, Nietsch L, et al. Pulse oximetry screening as a
complimentary strategy to detect critical congenital heart defects. Acta
Paediatr. 2009;98:682-6.
11. Koppel LR, Druschel CM, Carter T, Goldberg BE,
Mehta PN, Talwar R, et al. Effectiveness of pulse oximetry
screening for congenital heart disease in asymptomatic newborns.
Pediatrics. 2003;111:451-5.
12. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer
AK. Pulse oximetry screening for critical congenital heart defects in
asymptomatic newborn babies: a systematic review and meta-analysis.
Lancet. 2012;379:2459-64.
13. Haq FU, Jalil F, Hashmi S, Jumani MI, Imdad A,
Jabeen M, et al. Risk factors predisposing to congenital heart
defects. Ann Pediatr Cardiol. 2011;4:117-21.
|
|
|
 |
|

