|
|
|
Indian Pediatr 2013;50:
847-852 |
 |
Outcome of Very Low Birth Weight Infants with
Abnormal Antenatal Doppler Flow Patterns:
A Prospective Cohort Study
|
|
CVS Lakshmi, G Pramod, *K Geeta, S Subramaniam,
#Marepalli B Rao,
$Suhas G Kallapur, and S
Murki
From the Departments of Pediatrics and *Obstetrics and Gynecology,
Fernandez Hospital, Boggulkunta, Hyderabad, Andhra Pradesh, India;
#Division of Biostatistics, Dept. of Environmental Health
University of Cincinnati, 3223, Eden Avenue, Cincinnati, Ohio 45267; USA
and $Divisions of Neonatology and Pulmonary Biology, Cincinnati
Children’s Hospital Medical Center, University of Cincinnati, 3333
Burnet Avenue, Cincinnati, Ohio 45229, USA.
Correspondence to: Dr Srinivas Murki, Consultant Neonatologist,
Fernandez Hospital, Boggulakunta, Hyderabad.
Email:
srinivas_murki2001@yahoo.com
Received: September 06, 2012;
Initial review: October 16, 2012;
Accepted: February 26, 2013.
PII:
S097475591200788
|
Background:
Fetal growth
restriction and abnormal Doppler flow studies are commonly associated.
Neonatal outcomes are not well known particularly in developing
countries, where the burden of the disease is the highest.
Objective: To determine outcomes of
preterm infants with history of absent/reversed end-diastolic umbilical
artery Doppler flow (AREDF) vs. infants with forward
end-diastolic flow (FEDF).
Design: Cohort study.
Setting: Tertiary care perinatal center in India.
Participants: 103 AREDF very low birth weight
(<1500 gm) (VLBW) infants and 117 FEDF VLBW infants were prospectively
enrolled.
Results: At 40 weeks adjusted post-menstrual age,
AREDF vs. FEDF group had a higher risk for death in the
NICU (12% vs. 1%), respiratory distress syndrome (33% vs.
19%), and cystic periventricular leukomalacia (12% vs. 1%). At 12-18
months corrected age, AREDF vs. FEDF group had a trend towards increased
risk for cerebral palsy (7% vs. 1%, P=0.06). After
logistic regression analysis, adjusting for confounders, AREDF was
independently associated only with mortality in the NICU.
Conclusion: AREDF is an independent predictor of
adverse outcomes in preterm infants in a developing country setting.
Keywords: India, Intrauterine growth restriction, Outcome,
Prognosis, Pre-eclampsia.
|
|
Intrauterine growth restriction can be caused by a
number of conditions but pregnancy induced hypertension and vascular
disorders of the placenta are among the most common etiologies
responsible for about 25-30% of IUGR [1]. Although the incidence of IUGR
is about 8% in the Western world [2], the prevalence in the developing
world is much higher at ~35% [3].
Although a number of different modalities are used
for fetal surveillance of IUGR, umbilical Doppler flow pattern is one of
the most widely used tests [4]. A number of observational studies have
reported outcomes in IUGR infants with abnormal antenatal Doppler flow
pattern [5-10]. However,
there are few studies [11,12] from the developing world, where the
global burden of the fetal growth restriction and preeclampsia is the
highest [3,13]. This information is essential to devise strategies for
reducing the rates of still-births/prematurity globally [14]. We
hypothesized that an absent or reversed end diastolic flow in umbilical
artery (AREDF) would be an independent predictor of adverse short-term
and long-term infant outcomes. We report the comparison of AREDF vs.
forward end-diastolic flow (FEDF) on comprehensive short-term outcomes
and long-term neurosensory and growth outcomes in preterm infants.
Methods
Parents of 238 very low birth weight (VLBW) infants
(<1500 gm birth weight) and gestation <35 weeks born consecutively
between the periods January 2007 to December 2008 at our referral
perinatal center were prospectively approached for informed consent for
enrolment at admission into the NICU. Infants with major congenital
malformations were excluded. Gestational age was determined by a first
trimester ultrasound scan, or by the mother’s last menstrual period.
Antenatal umbilical artery Doppler flows (Voluson, Philips and Logic Q
machines) were measured in pregnant women less than 35 weeks of
gestation and reported as forward, absent or reversal of flow during
diastole. The indications for Doppler studies were (a) evidence
of growth restriction on serial scans (based on Mediscan charts, Chennai
[15]), (b) pregnancy induced hypertension, and (c) history
of intrauterine death in a previous pregnancy. The umbilical artery
Doppler velocimetries reported in the study were those obtained closest
to delivery. The study population was divided into two cohorts viz.,
AREDF group comprising VLBW infants with absent or reversed
end-diastolic flow velocities in the umbilical artery; and FEDF group
with VLBW infants with forward Doppler flow velocity in umbilical artery
and those in whom antenatal Doppler studies were not indicated.
The primary outcomes included: Composite outcome of
death or major neuro-morbidity at 12-18 months of corrected age, defined
as presence of cerebral palsy or visual or hearing impairment. The
secondary outcomes included morbidities common in preterm infants. The
diagnosis of cerebral palsy was made by clinical examination by
experienced physicians blinded to the antenatal Doppler studies. Hearing
impairment was defined as any degree of hearing loss requiring the need
for hearing aids.
The antenatal details of study infants were collected
retrospectively from a computerized database and patient medical
records. All enrolled infants were followed up weekly/biweekly till they
were 40 weeks of corrected postmenstrual age, and then at 3,6,9,12 and
18 months of corrected age for growth and neurological assessment in the
high-risk neurodevelopmental follow-up clinic. At 40 weeks of corrected
postmenstrual age, each infant had a cranial ultrasound and a brain stem
evoked response audiometry. Growth was evaluated by measuring the
weight, head circumference and length by a trained nurse and plotted on
the Indian Academy of Pediatrics growth charts. Neurological assessment
was done by experienced physicians using the Amiel-Tison method [16].
All measurements were performed by investigators blinded to antenatal
studies.
Statistical analysis: Outcome variables were
compared between the study and the control groups. Statistical analyses
were performed by using SPSS (Version 16.0 for Windows, SPSS Inc.,
Chicago, IL) followed by the R package (Version 13.2.1). Fisher’s exact
test was used for categorical variables and for continuous variables the
student t-tests (normally distributed data) or Mann-Whitney U tests
(data not distributed normally) were used. Significance was accepted at
P<0.05. For multivariate analyses, initial exploration of
associations were performed using classification tree and random forest
methodology (not reported). Based on the initial exploratory analysis,
several responses were modeled as predictors of outcome of interest. The
odds ratio of a predictor adjusted for the presence of the other
predictors along with 95% confidence interval is reported.
We estimated that a sample size of 88 patients in
each group would provide 80% power at 95% confidence level to detect a
4-fold difference in risk of the primary outcome (death or major
neuromorbidity) between the groups.
Results
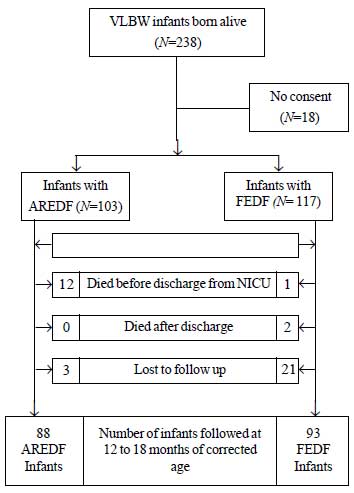
238 VLBW infants fulfilled the eligibility criteria.
Of these, 220 infants were analyzed for short-term outcomes. Long-term
outcomes were evaluated in 181 infants for growth and neurological
outcomes (Fig. 1). Compared to the AREDF group, more
infants in the FEDF group were lost to follow up (3.3% vs 18.6%,
P=0.001).
 |
|
Fig. 1 Study flow chart.
|
Although the degree of prematurity did not differ,
the infants in the AREDF group were smaller compared with FEDF group.
Expectedly, more infants in the AREDF group were growth restricted at
birth. Delivery by Caesarean section, and oligohydramnios was
significantly higher in the AREDF group compared with the FEDF group. (Table
I).
TABLE I Baseline Characteristics of Study Infants
|
Variable |
AREDF group
|
FEDF group
|
|
N=103 |
N= 117 |
|
*Birth weighta (g)
|
1095
|
1260
|
|
(951-1288) |
(1080-1400) |
|
Gestationa (wk)
|
31 (30-33) |
31 (30-32) |
|
†Birth weight <1000g |
32 (31) |
20 (17) |
|
Gestation <30 wk
|
22 (21) |
24 (21) |
|
Males
|
52 (50) |
47 (40) |
|
*IUGR
|
59 (57) |
36 (31) |
|
Apgar scores (5min) |
8 (7-8) |
8 (7-8) |
|
#Cesarean delivery
|
103 (99) |
103 (88) |
|
*Singleton pregnancy
|
98 (95) |
79 (68) |
|
Antenatal steroids
|
95 (92) |
102 (87) |
|
Maternal age, mean (SD) |
27.2 (4.4) |
26.7 (4.7) |
|
PIH |
77 (75) |
92 (79) |
|
$Oligohydramnios
|
35 (34) |
25 (21) |
|
PROM |
1 (1) |
21 (18) |
|
#Preterm labor
|
2 (2) |
17 (15) |
|
Data shown as amedian (inter-quartile range), rest as n (%); *
P=0.001; #P=0.001; $P=0.05; †P=0.02; P≤0.05;
AREDF: absent/reversed end-diastolic umbilical artery Doppler
flow; FEDF=forward end-diastolic flow; IUGR=Intrauterine growth
restriction; PIH: Pregnancy induced hypertension; PROM: Preterm
rupture of membranes. |
Short-term outcomes: More infants had
hospital deaths in the AREDF group compared to the FEDF group (Table
II). Need for resuscitation at birth was similar between the groups,
but the incidence of respiratory distress syndrome (RDS) was higher in
the AREDF vs FEDF group. There was a tendency to need more
respiratory support in the AREDF vs FEDF group. However both the
groups were comparable for morbidities such as patent ductus arteriosus,
neonatal jaundice, chronic lung disease, and retinopathy of prematurity.
TABLE II Short-term Outcome of Study Infants
|
Outcome |
AREDF (n=103)
|
FEDF (n=117) |
P value |
|
Mortality
|
12 (12) |
1 (1) |
0.001 |
|
Delivery room resuscitation
|
16 (16) |
23 (20) |
0.48 |
|
Hypoglycaemia
|
8 (8) |
4 (4) |
0.23 |
|
Respiratory distress syndrome
|
33 (33) |
21 (19) |
0.02 |
|
Continuous positive airway pressure
|
32 (32) |
23 (20) |
0.06 |
|
Conventional ventilation
|
34 (34) |
26 (23) |
0.09 |
|
Necrotizing enterocolitis (³ Bell stage IIa)
|
15 (15) |
9 (8) |
0.13 |
|
Culture positive sepsis
|
24 (24) |
15 (13) |
0.051 |
|
Hemodynamically significant Patent ductus arteriosus
|
5 (5) |
12 (11) |
0.20 |
|
Chronic lung disease (supplemental O2 at 28d)
|
1 (1) |
3 (3) |
0.62 |
|
Retinopathy of prematurity (³stage II)
|
15 (15) |
11 (9) |
0.40 |
|
Abnormal cranial ultrasound
|
17 (17) |
13 (11) |
0.42 |
|
Cystic periventricular leukomalacia
|
10 (12) |
1 (1) |
0.004 |
|
Time to reach full feedsa (d) |
8 (6-10) |
7 (4-8) |
0.001 |
|
Duration of hospitalizationa (d) |
20 (14-29) |
15 (11-71) |
0.03 |
|
Data shown as amedian (inter-quartile range); Rest as n(%);
AREDF: absent/reversed end-diastolic umbilical artery Doppler
flow; FEDF= forward end-diastolic flow. |
Of the 17 infants with abnormal cranial ultrasounds
in the AREDF group, leucomalacia and the others had grade III-IV
intraventricular hemorrhage (Table II).
Long term outcomes: The odds for the combined
outcome of death or cerebral palsy (n=18,17% vs n=4,
3%, OR 5.9, 95% CI 1.9 to 25) was 5.9 times higher in the AREDF group
compared to the FEDF. Also AREDF infants showed a trend toward
higher risk for developing cerebral palsy compared to the FEDF infants (n=6,7%
vs n=1,1%: P=0.06). None of the infants in either
group were blind or had deafness. There were also no differences between
the groups in the incidence of microcephaly, short stature or poor
weight gain.
On logistic regression analysis for the short-term
outcomes after adjusting for ELBW (birth weight<1000g) and IUGR status,
AREDF continued to have an independent association with neonatal
mortality (OR 9.8, 95% CI 2.1- 46.4) and RDS (OR 2.4, 95% CI,1.1-5.0).
For the long-term outcomes, AREDF had an independent association with
the composite outcome of death or cerebral palsy (OR 8.4, 95% CI 2.3-
30.5) but not cerebral palsy or microcephaly alone.
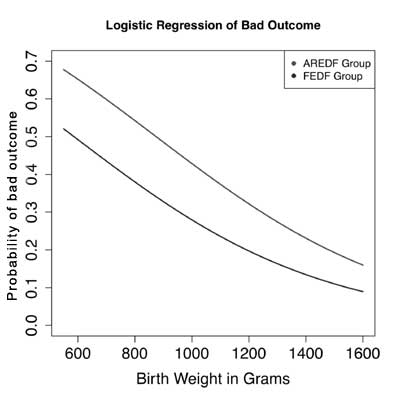
To better capture the contribution of AREDF in
neonatal outcomes, we examined all predictors of bad outcome in the
NICU. Since there were very few cases of chronic lung disease in this
population, bad outcome was defined as death or cystic periventricular
leukomalacia, or culture positive sepsis or necrotizing enterocolitis.
The only predictors that were significant were birthweight (P=0.01)
and Doppler flow status (P=0.05). To better model the
relationship between the predictors and bad outcome as a response, a
logistic regression curve of the probability of bad outcome as a
function of birth weight adjusted for AREDF or FEDF was fitted (Fig.
2). The preterm infants in the AREDF group had a consistently higher
probability of a bad outcome compared to the FEDF group with the
disadvantage being more pronounced at lower birth weights.
 |
|
Fig. 2 Relationship between
birth-weight, umbilical Doppler flow patterns and outcome. Bad
outcome (defined as death or periventricular leukomalacia, or
culture positive sepsis or necrotizing enterocolitis); AREDF:
Absent/reversed end-diastolic unbilical artery doppler flow;
FEDF: Forward end-diastolic flow.
|
Discussion
In this cohort of moderately preterm infants with a
history of fetal growth restriction or exposure to pre-eclampsia,
demonstration of antenatal absent or reversed end-diastolic flow in the
umbilical artery was shown to increase the risk for neonatal death. This
study was specifically designed to prospectively compare outcomes after
AREDF vs FEDF, the gestational ages between the groups were
comparable, and the numbers of infants were relatively large permitting
meaningful comparisons. We evaluated both short-term and long-term
outcomes comprehensively with follow-up rates in excess of 90%, all
infants were enrolled in a 2-year time-span from a single-perinatal
center minimizing the confounding of changing or differing management
practices on the outcomes, and the groups were relatively homogenous in
that the underlying diagnosis was PIH in a great majority. To our
knowledge, the present study is the largest and the most comprehensive
report on the contribution of abnormal Doppler flow patterns, and IUGR
to outcomes in Indian preterm infants.
The higher neonatal mortality and morbidity in
infants with history of AREDF noted in this study is similar to previous
studies [17-21]. In comparison with these older studies, we had higher
numbers of infants with absent or reversed end-diastolic flow and the
gestational ages in both the groups were comparable. Consistent with the
reported literature, findings from the present study confirm that
birthweight and gestational age are more potent predictors of short-term
adverse neonatal outcomes in infants with IUGR, compared to Doppler flow
patterns. Interestingly, our data clearly demonstrated that despite
birth weight being a potent predictor of poor neonatal outcomes, the
diagnosis of AREDF had an independent adverse impact at all birth
weights with a more pronounced effect at lower birth weights.
For long-term outcomes, our study showed an
independent association of AREDF with the composite outcome of cerebral
palsy or death in infancy. This effect was largely due to increased
neonatal deaths. Interestingly, although the rate of PVL at term
gestation was higher in the AREDF group, this did not translate into an
increased risk for adverse neuromorbidity (cerebral palsy tended to be
more common in the AREDF group). A factor that may explain the lack of
adverse neurological outcomes despite increased rates of PVL is
developmental plasticity in the preterm [22]. In this regard, infants in
the early delivery arm of a randomized trial evaluating early vs.
delayed delivery in IUGR infants had an increased risk for adverse
neurodevelopmental outcomes at two years that was not sustained at
school age [23,24]. Studies of neurodevelopmental outcome in infants
with IUGR and abnormal Doppler flows have reported inconsistent
outcomes. Studies with smaller numbers of infants have reported adverse
neurological outcomes [9,10,25], while others failed to demonstrate
neurologic impairments [8,26]. These discrepancies largely appear to be
due to different patient populations and the degree of prematurity
appears to be a predominant determinant of adverse long term
neurological outcomes rather than abnormal Doppler flow patterns
[5,27,28].
The variables of birthweight, gestation, IUGR and
Doppler flow patterns are inevitably interlinked. In this regard a
randomized trial (GRIT trial) evaluated trade-offs between immediate
vs. delayed delivery in the management of preterm infants ~28 weeks
gestation with fetal growth restriction [29]. The immediate delivery
group had higher neonatal deaths but the stillbirth rate was higher in
the delayed group. Adverse neurological outcome at 2 years was more
common in the earlier delivery group, but these handicaps did not
persist at school age [23,24]. The prevalent practice at our study site
is to give maternal glucocorticoids and deliver infants within 48 hours
after demonstration of absent/reversed end-diastolic flow, similar to
the early delivery arm of the GRIT trial. Despite the immediate
delivery, fetuses with AREDF had an increased mortality and morbidity in
our study, suggesting that the umbilical Doppler changes may be a late
finding in the pathophysiology of fetal compromise in IUGR and pre-eclampsia
[27]. Alternatively, the fetuses in our study may have been sicker than
previously reported. Regardless, the findings are informative for
clinicians managing these high risk pregnancies .
Despite several strengths of our study, some
weaknesses were apparent. The study population was entirely from a large
referral perinatal center with a higher rate of IUGR and pre-eclampsia
than the general population. Lost to follow up was significantly higher
in the forward flow group. The study was not randomized. Therefore the
findings of the study may not be generalizable. We did not evaluate
multiple different ultrasound measurements of fetal well-being, because
umbilical artery doppler studies are the most commonly used modality at
most perinatal centers dealing with high risk pregnancies.
In a cohort of moderate preterm delivery with IUGR or
maternal pre-eclampsia, absent or reversed end diastolic umbilical
arterial blood flow independently increased the risk for neonatal
mortality, and had a trend towards increased incidence of cerebral palsy
at 12-18 months corrected age.
Acknowledgements: Professors Alan H. Jobe
(Cincinnati) and John P. Newnham (Perth) for review of the manuscript
and helpful suggestions.
Contributors: All the authors have written,
designed and approved the study.
Funding: NIH HD-57869 to SGK;
Competing interests: None stated.
|
What is Already Known?
•
Fetal growth restriction and abnormal Doppler flow studies
are commonly associated.
• Neonatal outcomes are not well known in
developing countries.
What This Study Adds?
•
Preterm IUGR infants with
antenatal abnormal umbilical artery doppler, are at increased
risk for immediate mortality and long term neurological
disabilities.
|
References
1. Brodsky D, Christou H. Current concepts in
intrauterine growth restriction. J Intensive Care Med. 2004;19:307-19.
2. Mandruzzato G, Antsaklis A, Botet F, Chervenak FA,
Figueras F. Intrauterine restriction (IUGR). J Perinat Med.
2008;36:277-81.
3. Bang AT, Baitule SB, Reddy HM, Deshmukh MD, Bang
RA. Low birth weight and preterm neonates: Can they be managed at home
by mother and a trained village health worker? J Perinatol.
2005;25:S72-81.
4. Figueras F, Gardosi J. Intrauterine growth
restriction: New concepts in antenatal surveillance, diagnosis, and
management. Am J Obstet Gynecol. 2011
5. Baschat AA, Viscardi RM, Hussey-Gardner B, Hashmi
N, Harman C. Infant neurodevelopment following fetal growth restriction:
Relationship with antepartum surveillance parameters. Ultrasound Obstet
Gynecol. 2009;33:44-50.
6. Schreuder AM, McDonnell M, Gaffney G, Johnson A,
Hope PL. Outcome at school age following antenatal detection of absent
or reversed end diastolic flow velocity in the umbilical artery. Arch
Dis Child Fetal Neonatal Ed. 2002;86:F108-14.
7. Torrance HL, Bloemen MC, Mulder EJ, Nikkels PG,
Derks JB, et al. Predictors of outcome at 2 years of age after
early intrauterine growth restriction. Ultrasound Obstet Gynecol.
2010;36:171-7.
8. Valcamonico A, Accorsi P, Sanzeni C, Martelli P,
La Boria P, et al. Mid- and long-term outcome of extremely low
birth weight (elbw) infants: An analysis of prognostic factors. J Matern
Fetal Neonatal Med. 2007;20:465-71.
9 Ley D, Tideman E, Laurin
J, Bjerre I, Marsal K: Abnormal fetal aortic velocity waveform
and intellectual function at
7 years of age. Ultrasound Obstet Gynecol 1996;8:160-65.
10 Marsal
K, Ley D: Intrauterine blood flow and postnatal neurological development
in growth-retarded
fetuses. Biol Neonate 1992;62:258-64.
11. Devendra A, Sadhana D, Prem S, Prema K.
Significance of umbilical artery doppler velocimetry in the perinatal
outcome of growth restricted fetuses. J Obst Gynaec India.
2005;55:138-43
12. Malhotra N, Chanana C, Kumar S, Roy K, Sharma JB.
Comparision of perinatal outcomes of growth-restricted fetuses with
normal and abnormal umbilical artery Doppler waveforms. Indian J Med
Sci. 2006;60:311-7.
13. Goldenberg RL, McClure EM, Macguire ER, Kamath
BD, Jobe AH. Lessons for low-income regions following the reduction in
hypertension-related maternal mortality in high-income countries. Int J
Gynaecol Obstet. 2011;113:91-5.
14. Gravett MG, Rubens CE, Nunes TM. Global report on
preterm birth and stillbirth (2 of 7): Discovery science. BMC Pregnancy
Childbirth. 2010;10:S2.
15. Suresh S, Thangavel G. Mathemetical modeling of
fetal growth, Part I: data collection and descrtiptive statistics. IJMU.
2003;3;35-41.
16. Amiel-Tison C, Ellison P. Birth asphyxia in the
fullterm newborn: Early assessment and outcome. Dev Med Child Neurol.
1986;28:671-82.
17 Eronen M, Kari A,
Pesonen E, Kaaja R, Wallgren EI, Hallman M: Value of absent or
retrograde end-diastolic flow
in fetal aorta and umbilical artery as a predictor of perinatal
outcome in pregnancy-induced
hypertension. Acta Paediatr 1993;82:919-24.
18
Karsdorp VH, van Vugt JM, van Geijn HP, Kostense PJ, Arduini D et al:
Clinical
significance of absent or reversed end diastolic velocity waveforms in
umbilical artery. Lancet 1994;344:1664-68.
19. Yoon BH, Lee CM, Kim SW. An abnormal umbilical
artery waveform: A strong and independent predictor of adverse perinatal
outcome in patients with preeclampsia. Am J Obstet Gynecol.
1994;71:713-21.
20. Deshmukh A, Neelu S, Suneeta G. Significance of
umbilical artery Doppler velocimetry in the perinatal outcome of the
growth restricted babies. J Obst Gynaec India. 2010;l60:38-43.
21. Bora A, Mukhopadhyay K, Saxena AK, Jain V, Narang
A. Prediction of feed intolerance and necrotizing enterocolitis in
neonates with absent end diastolic flow in umbilical artery and the
correlation of feed intolerance with postnatal superior mesenteric
artery flow.J Matern Fetal Neonatal Med. 2009;22:1092-6.
22. Kostovic I, Judas M. Prolonged coexistence of
transient and permanent circuitry elements in the developing cerebral
cortex of fetuses and preterm infants. Dev Med Child Neurol.
2006;48:388-93.
23. Thornton JG, Hornbuckle J, Vail A, Spiegelhalter
DJ, Levene M. Infant wellbeing at 2 years of age in the growth
restriction intervention trial (grit): Multicentred randomised
controlled trial. Lancet. 2004;364:513-20.
24. Walker DM, Marlow N, Upstone L, Gross H,
Hornbuckle J, et al. The growth restriction intervention trial:
Long-term outcomes in a randomized trial of timing of delivery in fetal
growth restriction. Am J Obstet Gynecol. 2011;204:31-39.
25. Spinillo A, Montanari L, Bergante C, Gaia G,
Chiara A, et al. Prognostic value of umbilical artery doppler
studies in unselected preterm deliveries. Obstet Gynecol.
2005;105:613-20.
26. Kirsten GF, Van Zyl JI, Van Zijl F, Maritz JS,
Odendaal HJ. Infants of women with severe early pre-eclampsia: The
effect of absent end-diastolic umbilical artery doppler flow velocities
on neurodevelopmental outcome. Acta Paediatr. 2000;89:566-70.
27. Baschat AA, Odibo AO. Timing of delivery in fetal
growth restriction and childhood development: Some uncertainties
remain. Am J Obstet Gynecol. 2011;204:2-3.
28. Walker DM, Marlow N. Neurocognitive outcome
following fetal growth restriction. Arch Dis Child Fetal Neonatal Ed.
2008;93:F322-5.
29. GRIT Study Group. A randomised trial of timed delivery for the
compromised preterm fetus: Short term outcomes and bayesian
interpretation. BJOG. 2003;110:27-32.
|
|
|
 |
|

