|
|
|
Indian Pediatr 2012;49: 721 -725 |
 |
Blood Levels of Pyrazinamide in Children at
Doses Administered Under the Revised National Tuberculosis
Control Program
|
|
V Roy, P Sahni, P Gupta, *GR Sethi and
#A
Khanna
From the Departments of Pharmacology, *Pediatrics, and
#Chest Clinic; Maulana Azad Medical College and Lok Nayak Hospital, New
Delhi, India.
Correspondence to: Dr Vandana Roy, Professor,
Department of Pharmacology, Maulana Azad Medical College and Associated
Hospitals, Bahadurshah Zafar Marg, New Delhi 110 002, India.
Email: [email protected]
Received: July 1, 2011;
Initial review: September 17, 2011;
Accepted: October 31, 2011.
Published online: January 17, 2012.
SII: S097475591100563-1
|
|
Objectives: To evaluate the blood levels, pharma-cokinetics and
pharmacodynamic indices of pyrazinamide (PZA) in children suffering from
tuberculosis, at doses administered under the weight band system of
Revised National Tuberculosis Control Program of India (RNTCP) of India.
Design: Prospective, open-label,
non-randomized single-dose study.
Setting: 20 children in the age
group 5-12 years attending out-patient tuberculosis clinic of a tertiary
hospital.
Outcome Measures: Blood
levels of pyrazinamide after single dose administration, as per the
weight band system of RNTCP.
Results: Group I (n=7)
included children who received pyrazinamide within the recommended 30-35
mg/kg dose (mean 31.9+0.8 mg/kg) and Group II (n=13)
included those who received a dose lower than 30 -35 mg/kg (mean
28.1±0.3 mg/kg). The Cmax (95% CI of difference 2.2, 13.2; P=0.008)
and AUC (95% CI of difference 28.6, 208.1; P=0.01) were
significantly lower in Group II. The duration of time for which the
concentration was maintained above 25 µg ml-1 was 4-8 h in Group I and
3-5.5 h in Group II (95% CI of difference 0.1, 2.0; P=0.03). The
half life, elimination rate constant, clearance and volume of
distribution were comparable in the two groups. The ratios of Cmax and
AUC to MIC (25 µg ml-1) in children were lower than that recommended for
PZA in adults.
Conclusions: Lower blood
concentrations are being attained in children receiving PZA doses under
the existing weight band system of RNTCP of India. The weight bands may
need to be revised and dose recommendations be based on pharmacokinetic
and efficacy data in children.
Key words: Children, India, Pharmacokinetics,
Pyrazinamide, RNTCP, Tuberculosis.
|
Revised National Tuberculosis
Control Program (RNTCP) of India recommends pyrazinamide (PZA)
administration at 30-35 mg/kg for intermittent, thrice weekly short
course chemotherapy regime for tuberculosis in adults [1]. Children are
administered drugs according to a Patient-wise box system under RNTCP
[1]. Although the practical advantage of this system for drug
administration cannot be doubted, there is a probability that children
may be getting inappropriate doses when calculated on body weight basis.
In the few clinical studies where intermittent
antitubercular regimens have been administered, higher doses of PZA in
the range of 50-70 mg/kg were used (2-4). A PZA concentration of 25 µg/mL
is considered low for thrice weekly administration [5]. Use of low doses
in intermittent therapy may lead to inadequate drug concentrations which
may contribute to treatment failure, relapse and drug resistance. Higher
doses on the other hand may contribute to hepatotoxicity [2,6,7). There
is lack of data in Indian children on the blood levels of PZA achieved
with the current patient-wise box system. We conducted this study to
observe the PZA blood levels achieved in children falling under Weight
band 1 and 2 of RNTCP.
Methods
An open-label, prospective, non-randomized single
dose study was conducted in children, suffering from tuberculosis
attending the Tuberculosis Clinic of Lok Nayak Hospital, New Delhi,
India. The study was approved by the Institutional Ethics Committee.
Written informed consent was obtained from the parents/guardians of all
patients.
Twenty children in the age group of 5-12 years, newly
diagnosed with pulmonary or lymph node tuberculosis, were enrolled in
the study. Diagnosis of tuberculosis was based on relevant clinical
history, physical examination, chest X-ray, Mantoux test and fine
needle aspiration cytology of accessible lymph nodes, wherever required.
Patients with hematological, hepatic and renal functions within the
normal range were included. Patients with severe tuberculosis requiring
hospital admission, concomitant presence of any other disease, and
history of concomitant or long term drug intake were excluded.
Enrolled patients were admitted one day prior to
study commencement, immediately on confirmation of the diagnosis. After
overnight fasting, a single dose of PZA was administered orally at 06:00
h. The PZA dose was as per the Patient-wise box system of the RNTCP
guidelines for treatment of tuberculosis. Children with weight between
6-10 kg (Weight Band 1) were given PZA 250 mg and children weighing
between 10 and 17 kg (Weight Band 2) were given PZA 500 mg. A standard
breakfast and lunch was administered 2 and 6 h after PZA administration,
respectively. Regular antituberculosis treatment began 24 h later.
Venous blood samples (2.5 mL) were collected at
0,1,2,4,6,8,12 and 24 h after PZA administration. Serum was separated
within 2 h of sample collection. A 1 mL sample of serum was
deproteinised and supernatant was stored at –20 ºC
for 24 h after which the assay was performed. Pyrazinamide was estimated
by the spectrophotometric method of Subbammal, et al. [4].
The PZA dose administered to individual patients was
converted to mg/kg dose and the patients were divided into two groups.
Children for whom the PZA dose was within the recommended 30-35 mg/kg
range (RNTCP for intermittent therapy) were included in Group I. Whereas
children for whom the PZA dose was not in the 30-35 mg/kg range were
included in Group II.
A single open compartment model was used to interpret
the serum concentrations of PZA using WinNonlin Professional Version 4.0
(Pharsight Corp, Mountain View, CA, USA). The calculation of peak serum
concentration (C max), time
to attain the peak concentration (Tmax),
area under the serum concentration vs time curve (AUC),
elimination half life (t1/2),
elimination rate constant (kel),
apparent volume of distribution (Vd)
and oral clearance (CL) is described elsewhere (9). The minimum
inhibitory concentration (MIC) of PZA for Mycobacterium tuberculosis
for pharmacokinetic pharmacodynamic (PKPD) parameter calculation was
considered as 25mg/mL [4,5]. The ratio of Cmax:MIC
and AUC:MIC were calculated.
The demographic characteristics, baseline
investigations and serum PZA concentrations were compared using GEE
population-averaged model. The pharmacokinetic parameters (C max,
Tmax, AUC0-24,
AUC, t1/2, Vd,
CL) and pharmacodynamic indices (Cmax:MIC,
AUC:MIC and the time duration for which serum PZA
concentration remained above 25 µg/mL) were compared using two-sample
t-test for unpaired data. For statistical analysis P value of
<0.05 was considered significant at a confidence interval of 95%. The
results are expressed as mean (standard error of the mean).
Results
All 20 subjects completed the study. In both the
groups, patients were comparable in their demographic profile (Web
Table I). Two patients were in weight band 1, and 18
in weight band 2. When calculated in mg/kg doses, it was observed that
only seven children received pyrazinamide dose in mg/kg as per the RNTCP
guidelines (Group I). The mean dose of PZA was 31.9±0.8
mg/kg
(30.3-35.7 mg/kg) in Group I and 28.1±0.3 mg/kg
(25-29.4 mg/kg) in Group II.
The mean serum PZA concentration in Group I at the
end of 1h was 38.4±1.7 µg/mL. At 2h, the PZA concentration increased to
49.4±2.8 µg/ml. After 2h, PZA concentrations declined gradually till 24h
(4.8±1.0 µg/mL). The mean serum PZA concentration in Group II at 1h was
33.0±0.9 µg/ml and increased to 41.7±1.2 µg/mL at 2h. The PZA
concentration declined gradually after 2h to 2.9±0.5 µg/mL at 24h (Fig.
1). The PZA concentrations were significantly lower in Group II up
to 6h (P=0.003).
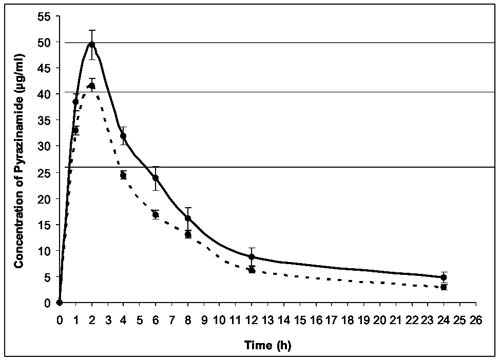 |
|
Fig. 1 Serum concentrations of
pyrazinamide over 24 h in Group I (n=7), (continuous line) and
Group II (n=13) (dotted line) (values expressed as Mean ± SEM)
in relation to MIC of 25, 40 and 50 µg/mL.
|
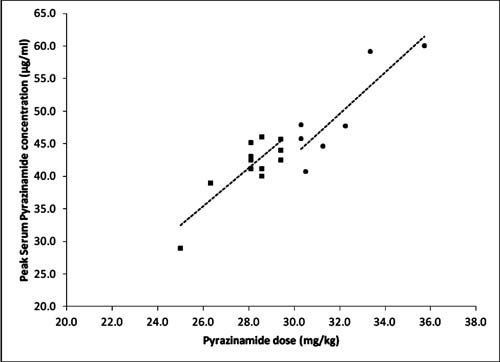 |
|
Fig. 2 Peak Serum pyrazinamide
concentration (Cmax) in relation to
the single oral pyrazinamide dose per kg body weight in Group I
(n=7, circles) and Group II (n=13, squares). The dashed lines
indicate a linear relation between the dose and Cmax in Group I
and II (R2= 0.76 and 0.7, respectively).
|
Pharmacokinetics: The C max
was achieved at 2h in all the patients. The mean Cmax
and AUC0-24 was
significantly less in Group II in comparison to Group I (Table
I). The serum PZA concentration was maintained above 25 µg/mL for
4-8 h in Group I. One patient in this group had levels above 25 µg/mL
for more than 6.5 h and another patient maintained this level for more
than 8 h. In Group II, the concentrations were maintained above 25 µg/mL
for not more than 3-5.5 h (P=0.03). The Cmax:MIC
and AUC:MIC ratios were also significantly lower in Group II. A linear
relationship was observed between the individual dose of PZA
administered and Cmax (Fig.
2). The other pharmacokinetic parameters i.e. t1/2,
Kel, Vd
and CL were comparable between the two groups.
TABLE I Pharmacokinetic Parameters (Mean±Sem) of Group I (Patients Receiving Appropriate mg/kg Dose)
and Group II (Not Receiving Appropriate mg/kg Dose) of Pyrazinamide as per Rntcp Guidelines
for Intermittent Therapy
| PK Parameter |
Group I(n=7) |
Group II(n=13) |
95% CI of difference |
P value |
| Cmax, µg/mL |
49.4±2.8 |
41.7±1.2 |
2.3, 13.2 |
0.008 |
| Tmax, h |
2.0 |
2.0 |
– |
– |
| AUC(0-24h), µg/mL h |
369.5±35.3 |
278.4±16.0 |
20.4, 161.6 |
0.01 |
| AUC, µg/mL h |
435.0±44.2 |
316.6±20.7 |
28.6, 208.1 |
0.01 |
| t1/2, h |
7.8±1.1 |
6.6±0.6 |
-1.2, 3.6 |
0.3 |
| Kel, h-1 |
0.12±0.03 |
0.12±0.01 |
-0.06, 0.06 |
0.9 |
| V, l/kg |
0.8±0.07 |
0.8±0.04 |
-0.2, 0.1 |
0.6 |
| CL, l/h/kg |
0.08±0.009 |
0.09±0.006 |
-0.04, 0.006 |
0.1 |
| Time > 25 µg/mL, h |
5.3±0.5 |
4.2±0.2 |
0.1, 2.0 |
0.03 |
| Cmax:MIC (25 µg mL-1) |
1.98±0.1 |
1.67±0.05 |
0.09, 0.5 |
0.008 |
| AUC:MIC (25 µg mL-1) |
17.4±1.8 |
12.7±0.8 |
1.1, 8.3 |
0.01 |
| SEM: standard error of
the mean; CI: Confidence Interval; Cmax: peak
serum drug concentration; tmax : time to achieve peak
serum concentration; AUC(0-24 h) : area under the serum
concentration time curve in 24 h; AUC : area under the serum
concentration time curve; t1/2 : elimination
half-life; Kel : elimination rate constant; V :
volume of distribution; CL : apparent clearance. |
Discussion
The RNTCP currently recommends two forms of drug
dosing for children. On one hand it recommends a dose of 30-35 mg/kg PZA
to children for thrice weekly therapy. On the other hand, it advocates
the Patient-wise box system which is resulting in PZA being administered
in a wide dose range of 25-45 mg/kg. In our study, only 35% children
received appropriate amounts of PZA in mg/kg basis. The dose per kg body
weight was an important determinant of PZA concentrations. This has been
observed previously also [8,9].
Pyrazinamide dosage recommendations for intermittent
therapy are based on two important factors, MIC and lag phase [3,10].
Although at present there is less data on PKPD correlates of PZA, it has
been observed that PZA blood concentration above 25 µg/mL is associated
with a prolonged duration of antimyco-bacterial effect for daily
administration [4]. However, for adequate killing with intermittent
dosing, a serum concentration of 20 and 25 µg/mL have been considered as
very low and low respectively [5,11]. We observed that patients who
received PZA lesser than the recommended 30-35 mg/kg dose could maintain
levels above 25 µg/mL for shorter duration of time. The ratios for MIC
25 µg/mL are below those suggested to be optimal [12].
For pyrazinamide, a C max
of 20-40 µg/mL after daily dose and 40-60 µg/mL after biweekly dose has
been recommended [13]. In our study, 18 children had Cmax
between 40-60.1 µg/mL. However, the PZA concentration fell below 40 µg/mL
within 4h of drug intake for many patients. Since the duration of time
for which blood concentration stays above MIC is important and we do not
know yet how much that duration should be, we cannot comment on the
adequacy of the PZA concentrations achieved. A critical PZA
concentration of 50µg/mL has also been defined based on inhibition of
≥ 95% wild
type isolates. The in vitro sterilizing effect of PZA was linked
to a PZA ratio of AUC: MIC with 90% maximal effect being achieved when
the ratio is 209. Patient simulation demonstrated that a dose of 15-30
mg/kg achieved this ratio in the epithelial lining fluid of only 15-53%
patients and dose more than 60 mg/kg performed better [14]. In view of
the above findings, the AUC:MIC ratio in our study appears inadequate in
all the patients.
A large inter-individual variation was observed in
the blood levels as has been reported earlier also [8,11,15]. The t 1/2
was longer and CL was slower in comparison to a population
pharmacokinetic study conducted in USA [11]. We are unable to explain
the difference. There are many differences with both the studies, such
as the PZA dose, frequency of administration, number of blood samples
taken and ethnicity of the patient population. In comparison to the
adult population, the t1/2
was longer, CL was slower and Vd
was larger [16]. It has been suggested that age has an inverse
relationship with t1/2 and Vd
and a direct relationship with CL (16,17). The Tmax
for PZA in children has been reported to be more than 2h. However, in
this study, it is 2h. This could be because there were no sampling
points between 2 and 4h.
Based on pharmacokinetic studies in children, it has
been suggested that PZA dose needs to be higher for children on a
bodyweight basis [11]. This observation is also based on the fact that
recent reports of outcome of childhood tuberculosis found a much poorer
treatment response than earlier studies [18]. WHO has also recommended
that intermittent therapy should not be used in children living in
settings with a high HIV prevalence [19].
Higher doses than the 30-35 mg/kg thrice weekly doses
followed in India under RNTCP are recommended by many professional
bodies (20,21). The recent WHO guidelines also recommend the use of PZA
in a daily dose of 30-40 mg/kg in children [19].
In the present study, the clinical outcome of
treatment given was not assessed. Since the ultimate test of inadequate
serum concentrations would be the effect on efficacy, this may be
considered a limitation of the study. The present study demonstrated
significantly low serum PZA levels in many children who received PZA in
accordance with the RNTCP patient-wise box system. This system while
easing the administration of drugs to children may not be adequate for
delivering appropriate amounts of antitubercular drugs, in this case
PZA.
Acknowledgements: Arbro Pharmaceuticals
Ltd, New Delhi for providing us with pure Pyrazinamide powder and Ms M
Kalaivani, Scientist, Department of Biostatistics, All India Institute
of Medical Sciences for statistical assistance.
Contributors: VR conceived and designed the
study. She was also involved in supervision of the study and analysis
and interpretation of results and preparation of the manuscript. She
will act as guarantor of the study. PS was involved in preparing the
study protocol, sample collection and biochemical analysis, calculation
of results and preparing manuscript. PG was involved in calculations of
results, statistical analysis and preparation of the manuscript. GRS and
AK contributed to planning the study, management of pediatric
tuberculosis patients and manuscript writing. The final manuscript was
approved by all authors.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• Pyrazinamide is
recommended in a dose range of 30-35 mg kg for thrice weekly
administration in the RNTCP to be delivered based on four weight
bands in children.
What This
Study Adds?
• Children may receive
pyrazinamide doses lower than the recommended range in weight
band I and II, resulting in lower blood levels and lesser
duration of time for which the pyrazinamide concentrations are
maintained above the minimum inhibitory concentration.
|
References
1. RNTCP Status Report. TB India 2007. Central TB
Division, DGHS, MoHFW. New Delhi: Government of India; 2007.
2. Ramakrishnan CV, Janardhanam B, Krishnamurthy DV,
Stott H, Subbammal S, Tripathy SP. Toxicity of pyrazinamide,
administered once weekly in high dosage, in tuberculous patients. Bull
World Health Organ. 1968;39:775-9.
3. Ellard GA. Absorption, metabolism and excretion of
pyrazinamide in man. Tubercle. 1969;50:144-58.
4. Subbammal S, Krishnamurthy DV, Tripathy SP,
Venkataraman P. Concentration of pyrazinamide attained in serum with
different doses of the drug. Bull WHO. 1968;39:771-4.
5. Perlman DC, Segal Y, Rosenkranz S, Rainey PM,
Peloquin CA, Remmel RP, et al. The clinical pharmacokinetics of
pyrazinamide in HIV infected persons with tuberculosis. Clin Infect Dis.
2004;38:556-64.
6. Ormerod LP. Analysis of the frequency of drug
reactions to antituberculosis drugs. Thorax. 1994; 49:433-4.
7. Singh J, Arora A, Garg PK, Thakur VS, Pande JN,
Tandon RK. Antituberculosis treatment induced hepatotoxicity: role of
predictive factors. Post Grad Med J. 1995;71:359-62.
8. Roy V, Tekur U, Chopra K. Pharmacokinetics of
pyrazinamide in children suffering from pulmonary tuberculosis. Int J
Tuberc Lung Dis. 1999;3:133-7.
9. McIlleron H, Willemse M, Schaaf HS, Smith PJ,
Donald PR. Pyrazinamide plasma concentrations in young children with
tuberculosis. Pediatr Infect Dis J. 2011;30:262-5.
10. Mitchison DA. Basic mechanisms of chemotherapy.
Chest. 1979; 76 (suppl):771- 81.
11. Zhu M, Burman WJ, Steiner P, Stambaugh JJ, Ashkin
D, Bulpitt AE, et al. Population pharmacokinetic modeling of
pyrazinamide in children and adults with tuberculosis. Pharmacotherapy.
2002;22:686-95.
12. Nueremberger E, Grosset J. Pharmacokinetic and
pharmacodynamic issues in the treatment of mycobacterial infections. Eur
J Microbiol Infect Dis. 2004;23:243-55.
13. Peloquin CA. Therapeutic drug monitoring in the
treatment of tuberculosis. Drugs. 2002;62:2169-83.
14. Gumbo T. New susceptibility breakpoints for first
antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic
and population pharmacokinetic variability. Antimicrob Agents Chemother.
2010;54:1484-91.
15. Gupta P, Roy V, Sethi GR, Mishra TK. Pyrazinamide
blood concentrations in children suffering from tuberculosis: a
comparative study at two doses. Br J Clin Pharmacol. 2008;65:423-7.
16. Lacroix C, Hoang TP, Nouveau J, Guyonnaud C,
Laine G, Duwoos H, Lafont O. Pharmacokinetics of pyrazinamide and its
metabolites in healthy subjects. Eur J Clin Pharmacol. 1989;36:395-400.
17. Kearns GL, Reed MD. Clinical pharmacokinetics in
infants and children: A reappraisal. Clin Pharmacokinet. 1989;17 (suppl):
29-67.
18. Te Water Naude JM, Donald PR, Hussey GD, Kibel
MA, Louw A, Perkins DR, et al. Twice weekly vs. daily
chemotherapy for childhood tuberculosis. Pediatr Infect Dis J.
2000;19:405-10.
19. Rapid advice: treatment of tuberculosis in
children. WHO 2010. WHO/HTM/TB/2010.13. Available from:http://whqlibdoc.who.int/publications/2010/9789241500449
_eng.pdf. Accessed on april 16, 2011.
20. CDC. Recommendations and Report. Treatment of
Tuberculosis. American Thoracic Society, CDC and Infectious Disease
Society of America. MMWR Morb Mortal Wkly Rep. 2003;52:1-77.
21. Ormerod LP, Citron KM, Darbyshire JH, Smith ML.
Chemotherapy and management of tuberculosis in the United Kingdom:
Recommendations of the Joint Tuberculosis Committee of the British
Thoracic Society. Thorax. 1990;45:403-8.
|
|
|
 |
|

