|
|
|
Indian Pediatr 2012;49: 707-709
|
 |
Introduction of Haemophilus Influenzae
type b (Hib) as Pentavalent (DPT-HepB-Hib) Vaccine in Two States
of India
|
|
Satish Kumar Gupta, *Stephen Sosler and *Chandrakant Lahariya
From United Nations Children Fund (UNICEF), Country
Office, New Delhi, India and *World Health Organization (WHO), Country
Polio Surveillance Project, New Delhi, India.
Correspondence to: Dr Satish Kumar Gupta, Health
Specialist, UNICEF, 73 Lodi Estate, New Delhi, India.
Email: sgupta@unicef.org
|
|
Haemophilus influenzae
type b (Hib) bacterium was estimated to have
caused 8.1 million cases of serious Hib diseases, and 371,000 deaths
globally in the year 2000 [1]. In India, an annual estimated 2.4 to
3.0 million cases and 72,000 deaths in under-5 children were
attributed to Hib diseases [1, 2]. Safe and effective vaccines to
prevent Hib diseases have been available for nearly two decades and
are being used globally. The National Technical Advisory Group on
Immunization (NTAGI) in India recommended the introduction of Hib
vaccine in the Universal Immunization Program (UIP) in 2008 [2].
From December 2011, Hib vaccine in combination with diphtheria,
pertussis, tetanus and hepatitis B has been introduced in UIP in
Kerala and Tamil Nadu states. A comprehensive technical review on
Hib diseases and vaccines was published in 2009 in this journal [2].
This paper provides an update on global Hib vaccine use, and reviews
the process and steps undertaken in India to introduce Hib-containing
pentavalent vaccine in Kerala and Tamil Nadu.
Hib Diseases
It is estimated that mortality due to Hib disease
contributes 4% of all annual under-5 deaths in India [1, 3, 4]. The
fastidious nature of the Hib bacterium and poor laboratory
infrastructure in developing country settings such as India makes
the diagnosis of Hib diseases and calculation of disease burden
extremely difficult. Moreover, a combination of limited access to
health services and poor health-seeking behavior by rural
populations results in many affected children never having the
opportunity of being correctly diagnosed or receiving appropriate
care [5]. Even for those children who do reach health facilities,
the increasing prevalence of antibiotic-resistance makes treatment
difficult [6, 7].
Hib Vaccines and Their Introduction
Hib vaccines are available in liquid and
lyophilized formulations and presented in monovalent format or
combined with other antigens such as DPT and/or hepatitis B
antigens. Hib vaccines have been shown to be cost-effective in both
developed and developing country contexts and are in use for more
than two decades. The World Health Organization (WHO) recommends
that Hib vaccines be included in routine infant immunization
programs of all countries [7]. By June 2011, Hib vaccine, in various
formulations, was included in the national immunization program of
170 countries in all regions of the world [8].
Hib vaccination also reduces nasopharyngeal
colonization with the bacterium, resulting in further reductions of
Hib disease incidence. In addition to the effects directly
attributed to the vaccine, there are important indirect effects
associated with Hib vaccines. Indirect benefits include herd
immunity and reductions in antibiotic resistance by preventing
disease and inappropriate use of antibiotics. These benefits have
been amply demonstrated by the post-introduction studies where near
elimination levels of Hib disease have been reached;
near-elimination of the disease occurred in both industrialized and
developing countries, even countries with moderate to low
immunization coverage rates [9-12].
Decision-making
In April 2008, the NTAGI Sub-committee on Hib
vaccine reviewed available literature and information related to
disease burden in India, vaccine availability, safety and efficacy,
and cost-effectiveness. Based on this information, the Sub-committee
recommended that Hib-containing pentavalent vaccine should be
introduced in the country [2,3]. In a subsequent NTAGI meeting held
in June 2008, the Sub-committee recommendations were discussed.
Based on the cumulative weight of supporting evidence, the
Sub-committee’s recommendation to introduce Hib-containing
pentavalent vaccine was endorsed [3]. Importantly, the Indian
Academy of Pediatrics (IAP) had already recommended in 2006 the use
of Hib vaccine for all children [13]. The use of Hib vaccines in the
private sector is widespread in India for almost a decade. Following
the recommendation of NTAGI, the Ministry of Health and Family
Welfare (MoHFW), Government of India (GoI), decided to introduce the
vaccine initially in two states.
Strategy for Vaccine Introduction
Government of India has introduced Hib as liquid
pentavalent vaccine (LPV) combined with DPT and HepB in 10-dose
presentation. The use of combination formulation has certain clear
programmatic advantages. First, the number of injections per
completed schedule will be less, consequently requiring fewer
syringes and generating less potentially hazardous sharps waste. In
addition, cold chain space will be saved as a single vial of LPV
replaces DPT and Hep B vials. LPV has been recommended for all
infants and will be given in a 3-dose schedule. The first dose is
given at 6 weeks of age or older followed by dose 2 after a gap of
at least 4 weeks and a gap of at least 4 weeks before dose 3 (Table
I). The vaccine is offered to all children younger than 1
year of age and the booster dose is not recommended in UIP in India
[2, 14].
TABLE I Immunization Schedule following Pentavalent Vaccine Introduction
|
Age |
Current schedule |
After introduction of
Pentavalent vaccine |
|
At Birth |
BCG, OPV-0, HepB-Birth dose |
BCG, OPV-0, HepB-Birth dose |
|
6 weeks |
OPV-1, DPT-1, HepB1 |
OPV-1, Pentavalent-1 |
|
10 weeks |
OPV-2, DPT-2, HepB2 |
OPV-2, Pentavalent-2 |
|
14 weeks |
OPV-3, DPT-3, HepB3 |
OPV-3, Pentavalent -3 |
|
16-24 months |
DPT-B1, MCV2, OPV-B1 |
DPT-B1, MCV2, OPV-B1 |
|
5-6 year |
DPT-B2 |
DPT-B2 |
To facilitate and ease program implementation,
Government of India policy states that LPV will be given to a
progressive birth cohort whereby all children who present for their
first dose of DPT (DPT 1) will be provided their first dose of LPV
(LPV 1). Infants who had already initiated their schedule of DTP +
HepB will complete the DPT and HepB vaccines schedule. In addition,
monovalent Hepatitis B vaccine will continue to be used for
birth-dose and DPT vaccines will continue to be used for 16-24
months and 5-6 years of age booster doses [2, 14].
Preparing for Vaccine Introduction
The operational guidelines and frequently asked
questions for the introduction of LPV in UIP were developed and
provided to Kerala and Tamil Nadu states for wider dissemination,
several months prior to the initiation of LPV use. State
immunization program managers were sensitized on the strategies and
principles of LPV introduction during a National level UIP review
meeting held in May 2011 in New Delhi. In addition, LPV introduction
training materials were produced for medical officers and health
workers.
Training and sensitization within states then
followed a cascade format. State level orientations and training
workshops were held and attended by district immunization officers,
medical college faculty and other stakeholders. District
authorities, program managers, and PHC-block medical officers were
sensitized. In turn, block medical officers trained frontline health
workers on key aspects related to LPV and its introduction.
Representatives of professional associations, such as IAP Indian
Medical Association (IMA), and other stakeholders were also
sensitized at various levels.
In synergy with trainings, information, education
and communication (IEC) material including frequently asked
questions, were prepared in local languages and widely disseminated.
A media-sensitization workshop on Hib diseases and vaccination was
conducted in each state just prior to LPV introduction. The program
was launched by the State Ministers of Health and other senior
health department officials in Kerala and Tamil Nadu.
Capitalizing on Opportunity
Lessons from the introduction of Hepatitis B
vaccine in 10 states of India in 2007-08 illustrated the
immunization system strengthening opportunities that introducing a
new vaccine affords. Likewise, the introduction of LPV has been used
to strengthen Kerala and Tamil Nadu immunization systems by
training/re-training health personnel on proper injection
techniques, assessing and correcting existing cold chain problems,
improving program monitoring and supervision, and enhancing
reporting of adverse events following immunization. In addition,
pre-introduction training phase emphasized the importance of
maintaining sufficient stocks of monovalent Hepatitis B and DPT
vaccines to ensure the application of birth-dose and DPT booster (at
16-24 months and 5-6 years of age).
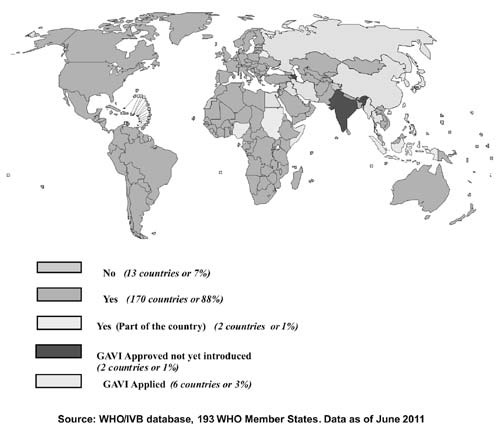 |
|
Fig. 1 Countries which have
introduced Hib vaccine in the National Immunization Program.
|
Moreover, the introduction of Hib-containing
pentavalent vaccine offers poorer families the opportunity to
provide the same life-saving protection to their children as
wealthier families who can afford vaccine services in the private
sector. Therefore, the introduction of Hib vaccine, free of cost in
the government system, helps to ensure equity in health service
availability – a stated objective of India’s National Health Policy
[15].
From a public health perspective, this is not a
trivial issue: Hib is one of the leading cause of bacterial
meningitis in India and a major cause of childhood pneumonia, the
largest killer of Indian children less than 5 years of age. It is
estimated that Hib disease prevention through vaccine use has the
potential to reduce India’s under-5 mortality rate by 4 percentage
points. The introduction of LPV in India is a major milestone and a
step forward to accelerate child survival in India, and progress
towards achieving national health goals and Millennium Development
Goal 4.
Competing interests: None stated.
Disclaimer: The view expressed in this paper
are those of the authors and should not be attributed to the UNICEF
and WHO.
References
1. Watt JP, Wolfson LJ, O’Brien KL, Henkel E,
Deloria-knol M, McCall N, et al. Burden of disease caused by
Haemophilus influenzae type b in children younger than 5
years: global estimates. Lancet. 2009; 374: 903-11.
2. Subcommittee of NTAGI. NTAGI subcommittee
recommendations on Haemophilus influenzae type b (Hib)
vaccine introduction in India. Indian Pediatr. 2009;46:945-54.
3. Government of India. Operational guidelines:
Introduction of Hib as Pentavalent Vaccine in Universal Immunization
program in India. Ministry of Health and Family Welfare, Govt. of
India, New Delhi, 2011.
4. United Nations Children’s Fund. Level and
Trends in Child Mortality: Report 2010. UNICEF and WHO, New York;
New York and Geneva: 2010.
5. National Family Health Survey (NFHS)
2005-2006, International Institute of Population Sciences, Mumbai,
India. Available at www.nfhsindia.org. Accessed on March 1,
2012.
6. World Health Organization. Introduction of
Haemophilus influenzae type b vaccine into immunization
programmes. World Health Organization, Geneva, 2000.
7. World Health Organization. WHO Position Paper
on Haemophilus influenzae type b conjugate vaccines. Weekly
Epidemiol Rec. 2006;81:445-52.
8. World Health Organization. WHO/ IVB database
of 193 WHO member states, as of June 2011
9. Cowgill KD, Ndiritu M, Nyiro J, Slack MP,
Chiphatsi S, Ismail A, et al. Effectiveness of Haemophilus
influenzae type b conjugate vaccine introduction into routine
childhood immunization in Kenya. JAMA. 2006;296:671-8.
10. Lee EH, Lewis RF, Makumbi I, Kekitiinwa A,
Ediamu TD, Bazibu M, et al. Haemophilus influenzae
type b conjugate vaccine is highly effective in the Ugandan routine
immunization program: a case-control study. Trop Med Int Health.
2008;13:495-502.
11. Baqui AH, El Arifeen S, Saha SK, Persson L,
Zaman K, Gessner BD, et al. Effectiveness of Haemophilus
influenzae type b conjugate vaccine on prevention of pneumonia
and meningitis in Bangladeshi children: a case-control study.
Pediatr Infect Dis J. 2007;26:565-71.
12. Watt JP, Levine OS, Santosham M. Global
reduction of Hib disease: what are the next steps? Proceedings of
the meeting Scottsdale, Arizona, September 22-25, 2002. J Pediatr.
2003;143: S163-87.
13. IAP guide book on immunization , IAP
Committee on Immunization 2007-2008, Indian Academy of Pediatrics,
Jaypee Brothers, New Delhi
14. Government of India. Pentavalent Vaccine.
Guide for Health Workers. Ministry of Health and Family Welfare,
Govt. of India, New Delhi, 2011.
15. National Health Policy 2002 (India), Ministry
of Health and Family Welfare. Available at http://mohfw.nic.in/nrhm/documents/national_health-policy_2002.pdf.
Accessed on March 1, 2012.
|
|
|
 |
|

