|
|
|
Indian Pediatr 2011;48:
697-701 |
 |
Evaluation of Cardiac Iron Load by Cardiac
Magnetic Resonance in Thalassemia |
|
Rashid Merchant, Aditi Joshi, Javed Ahmed, *Pradeep Krishnan and *Bhavin
Jankharia
From the Department of Pediatrics, Dr Balabhai Nanavati
Hospital, Mumbai, India;
and *Piramal Diagnostics, Mumbai, India.
Correspondence to: Dr Rashid H Merchant, 501, Ran gmahal
5th floor, 2 Mount Mary Road, Bandra (West),
Mumbai 400 050, India.
Email: deandoc2000@hotmail.com
Received: January 16, 2010;
Initial review; March 02, 2010;
Accepted: August 23, 2010.
Published online: 2010,
November 30.
PII: S097475591000044-1
|
Objective: To quantify myocardial iron stores by Cardiac Magnetic
Resonance (CMR) .
Design: Prospective cohort study.
Setting: Thalassemia center in a teaching hospital.
Participants: 60 transfusion dependant thalassemia
major patients and 10 controls during 2008-2009.
Methods: MRI T2* for cardiac iron load and cardiac
functions was performed on a 1.5 Tesla Siemens Sonata machine using the
thalassemia tools software. Ejection fraction (EF) was measured using
standard CMR sequence and EF <56% considered as cardiac dysfunction.
Quantification of iron deposition was categorized as T2* <10 milliseconds
(ms) as high risk, 10-20 ms as intermediate risk and >20 ms as low risk.
Simultaneous liver iron T2* values were categorized into normal i.e. >6.3
ms, mild iron overload 6.3 - 2.7 ms , moderate iron overload 2.7- 1.4 ms
and severe iron overload <1.4 ms. Pretransfusion serum ferritin levels
were simultaneously determined. Data was analyzed by paired and unpaired
t test of mean.
Results: Of 60 patients, 50% had no cardiac
siderosis; 33.3% had mild to moderate and while 16.7% had severe cardiac
siderosis . In contrast, only 8.3% had normal liver iron values, 55.7% had
mild to moderate and 36% had severe iron stores. The mean serum ferritin
of all 60 cases was 3528.6 ± 1958.6 ng/mL. There was a statistically
significant difference in the mean cardiac T2* of patients (23.45 ± 13.4
ms) as compared to controls (32.67 ± 2.68 ms) (P<0.01).
Conclusions: Thalassemia patients had significantly
higher cardiac iron stores as compared to controls. Serum ferritin and
liver iron values did not correlate with cardiac iron values. Three of 10
patients <10 years showed evidence of myocardial siderosis.
Key words: Cardiac siderosis, Magnetic resonance imaging,
Myocardial dysfunction, Thalassemia
|
|
Despite the availability of iron chelation, cardiac iron overload accounts
for most deaths in thalassemia major. Without adequate iron chelation
myocardial siderosis develops within the first decade of life and leads to
progressive cardiomyopathy. Measurement of cardiac iron presents a major
challenge as neither serum ferritin nor liver iron, are reliable
indicators of cardiac iron overload. Cardiovascular magnetic resonance (CMR)
is the established gold standard for quantifying cardiac iron and
ventricular function [1].
Measurement of cardiac iron by T2* on MRI provides
useful information on severity of myocardial siderosis [1]. T2* gradient
echo measures decay in signal intensity as echo time of images
progressively increases. This rate of decay is enhanced in presence of
iron deposition and hence increased iron levels reduce T2* values. Cardiac
T2* value <20
milliseconds (ms) is indicative of iron overload and below this value
there is progressive decline in left ventricular function. Values of <10
ms are considered suggestive of severe cardiac siderosis. Thus, T2* can be
used as a guide to severity of cardiac risk i.e. >20ms as low, between
20-10 ms as intermediate and <10 ms as high risk [2].
The aim of this study was to assess cardiac iron
overload and evaluate cardiac function using a single slice multi echo T2*
MR sequence and cine imaging in patients with thalassemia major.
Methods
60 regularly transfused thalassemia major patients (34
males) ages ranging from 6 to 26 years (mean 17 years) and 10 healthy, age
- matched controls were studied. All patients were on regular blood
transfusion administered at 2-4 weekly intervals to maintain the
pretransfusion hemoglobin of at least 8 g/dL. Informed consent was
obtained from patients or guardians and the hospital ethical committee
approved the study protocol. Of 60 cases, 41 were receiving oral
deferiprone (L1) at 60-65mg/kg/day, 16 receiving combination of L1
(60-65mg/kg) along with desferrioxamine (DFO) subcutaneously (30-35mg/kg)
for 5 days a week, while 3 were receiving only DFO. All cases tolerated
chelation therapy well.
T2* for cardiac iron was performed using 1.5 Tesla
Siemens Sonata machine and all patients were scanned using a single 8 mm
thick, short-axis, mid-left ventricle slice acquired at 8 different echo
times. Systolic and diastolic ventricular volumes and EF were measured
using a standard, reproducible CMR sequence and a semi-automated software,
as per published norms [3,4]. EF of <56% was considered to represent
cardiac dysfunction. Serum ferritin levels were estimated by ELISA from a
pretransfusion sample. Although hepatic iron stores were simultaneously
measured using a similar technique to the heart with a non-ECG gated
multi-echo sequence, it did not form part of the primary study analysis.
Data are presented as mean ± standard deviation (SD).
Variables were analyzed by paired and unpaired t test of means to
determine statistical differences and P value <0.05 was considered
statistically significant for any given measure.
No attempt was made in this study to analyze the
effects of iron chelation on extent of cardiac iron overload.
Results
Patient demographics, ventricular parameters, cardiac
T2* and liver T2* values are shown in Table I. The mean
blood transfusion requirement was 219.42 ml/kg/year with a range of
180-266 mL/kg/year. The mean serum ferritin of all 60 cases was 3528.6 ±
1958.6 ng/mL.
TABLE I
Demographics, Hemodynamics, Cardiac and Liver MRI T2* Results (N=60)
|
Parameter |
Range |
Mean |
|
Age (y) |
6-26 |
17 |
|
Serum Ferritin ng/mL |
133-7500 |
3528.6 ± 1958.6 |
|
Cardiac T2* ms |
6.24-69.9 |
23.45 ± 13.4 |
|
<10 y (n=10) |
12.68-50.62 |
25.82 |
|
10-20 y (n=33) |
6.24-69.19 |
22.32 |
|
>20 y (n=17) |
8.22-44.84 |
24.26 |
|
LVEF (%) |
50.9-76.2 |
62.87 |
|
EDV (mL) |
37.9-148.7 |
86.1 |
|
ESV (mL) |
10.9-69.9 |
32.56 |
|
Liver T2* ms |
0.93-16.2 |
2.7 |
|
<10 y (n=10) |
1.07-5.09 |
1.92 |
|
10-20 y (n=33) |
0.93-12.65 |
2.46 |
|
>20 y (n=17) |
1.09-16.2 |
3.71 |
|
LVEF- left ventricular ejection fraction; EDV-end diastolic volume,
ESV-end systolic volume; ms – milliseconds. |
 |
|
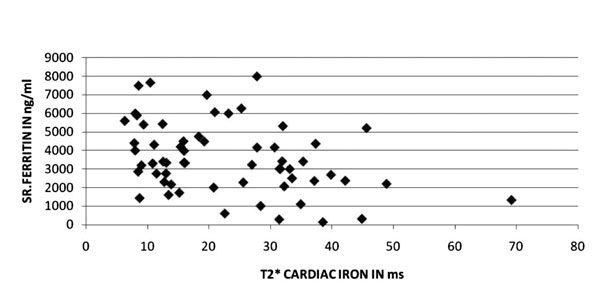
Fig. 1 Relation between serum ferritin and
cardiac T2*. |
 |
|
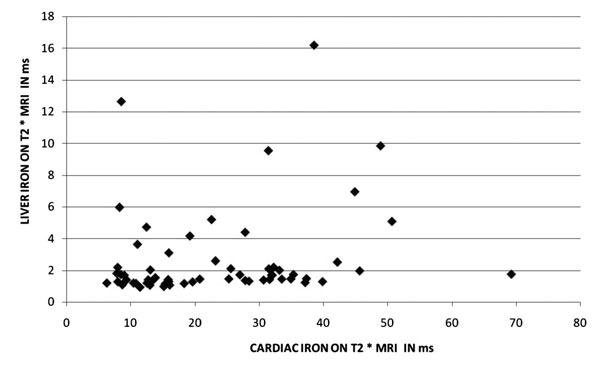
Fig. 2 Relationship between liver and
cardiac T2*. |
Cardiac T2* values in relation to serum ferritin and
liver T2* are depicted in Fig. 1 and 2, respectively.
Of 60 subjects, 50% had normal cardiac iron load (>20 ms), 33.3% had mild
to moderate cardiac siderosis (20-10 ms) and 16.7% had severe cardiac
siderosis (<10 ms). In contrast, only 8.3% subjects had a normal liver
iron (>6.3 ms), 55.7% had mild to moderate liver iron load (6.3-1.4 ms)
and 36% had severe liver iron load (<1.4 ms). Moderate to severe liver
iron involvement was noted in 81% of patients. Abnormal cardiac T2* (<20
ms) was found in 33.3%, 54.3% and 37.5% in the age group of <10 , 10-20
and >20 years, respectively. There was a statistically significant
difference (P<0.01) in the mean cardiac T2* value of the 60
patients (23.45 ± 13.4ms) as compared to that of 10 controls (32.67 ± 2.68
ms). Liver iron correlated poorly with myocardial iron concentration (Fig.
3). In the present study, there was no difference in the SF or
liver iron T2* levels between those with or without detectable cardiac
siderosis.
Discussion
In thalassemia, iron overload occurs as a result of
repeated blood transfusions and excessive iron absorption from the gut
[1,3]. The human body has no mechanism of excreting excess iron, which
gets deposited into body tissues, including cardio-myocytes, leading to
iron induced cardiac disease. When the iron binding capacity of
transferrin is saturated, free iron appears as non transferrin bound iron.
This toxic labile cellular iron causes generation of oxygen free radicals
resulting in oxidative stress, leading to impaired function of the
mitochondrial respiratory chain of the myocardium and to cardiac
dysfunction [1,3]. As iron accumulates in the myocardium, there is little
effect on its contractile function until a critical threshold is reached
above which rapid deterioration can occur. This explains why abnormal
systolic function is a late sign of cardiac toxicity in thalassemia.
Severe myocardial siderosis causes a toxic dilated cardiomyopathy that can
be reversed if aggressive chelation is begun early [5]. Recently,
non-invasive assessment of myocardial iron with magnetic resonance
relaxometry has been evaluated [1].
Iron deposits cause magnetic field inhomogeneity and
shorten the relaxation parameters T1, T2 and T2*. Prior to the
introduction of cardiac T2* quantification there was no accurate measure
to predict risk of iron induced cardiac disease in TM and the risk of
developing heart failure was estimated by sequential SF or liver iron
concentration. Evidence is also available from animal studies [6] that
cardiac iron load correlates well with cardiac T2*, and work is underway
to provide a calibration of T2* for myocardial iron load in human [7].
Measurement of T2* is now widely used for the heart as it is easily
combined with cardiac gating, is fast and robust, and is sensitive to iron
deposition [4].
In the present study, we have shown that 50% of the
patients had significant cardiac iron overload (T2* <20ms). The prevalence
of severe cardiac iron overload (T2*<10 ms) in our study population was
16.7%. Even in patients under the age of 10 years, a high degree of iron
loading was found with 33.3% having a myocardial T2* <20ms. There were 10
patients under 10 years. Three of these had T2* <20 ms, the youngest one
being a 6 year old with T2* value of 12.7ms, suggesting very early onset
of cardiac siderosis.
Liver iron overload was found in 93% of the patients
with 53% having evidence of severe hepatic siderosis (T2*<1.4 ms). Despite
this, only 2 patients (3.3%) had evidence of impaired LV systolic function
(EF <56%). There was no difference in the SF or liver T2* levels between
those with or without detectable cardiac siderosis. To confirm that these
findings were not spurious, the mean SF values for 12 months prior to the
scan were compared to cardiac T2* and there was no significant
correlation. The mean value for cardiac T2* in the normal healthy controls
was 32.67 ± 2.68ms and there was a significant difference in mean cardiac
T2* values (P<0.01) between controls and the study population.
As expected, there was a significant difference in mean
cardiac T2* values between controls and study population. These findings
compare well with previous data in Caucasians [1]. Normal ranges for T2*
have been determined from observational data in normal population and
patients with thalassaemia. The threshold of T2* <20 ms as an index of
cardiac risk is widely accepted with increasing risk of heart failure and
arrhythmias when T2* is below 10ms [8]. It is important to note that these
ranges are only applicable at a field strength of 1.5 Tesla.
In our study population, the age, transfusion
requirements, and compliance to chelation therapy were so tightly
connected that it was not possible to decipher which of these individual
factors was mainly responsible for cardiac siderosis. While T2* gives an
accurate impression of cardiac iron, it is not yet possible to predict the
exact myocardial iron concentration from the T2* values we determined. A
full calibration would require correlation with myocardial tissue.
Although myocardial tissue can be obtained from myocardial biopsy, this is
a difficult, invasive procedure with risk of life threatening
complications. There is also the issue of non homogenous myocardial iron
deposition and therefore biopsy is not clinically useful as an index of
cardiac iron load [8-12].
The majority of our patients with significant cardiac
siderosis would have been considered at low risk for cardiac disease on
the basis of serum ferritin level alone. We have confirmed that liver iron
correlates poorly with myocardial iron concentration and in agreement with
observational data from other studies, we found no difference in the SF or
liver iron T2* levels between those with or without detectable cardiac
siderosis [1,13]. The unreliable predictive value of SF measurements has
made heart disease difficult to detect and cardiac failure and arrhythmias
remain the leading cause of death [5,14]. SF is not a sensitive predictor
of subclinical cardiac disease and cardiac deaths can occur even with SF
levels <2500 ng/mL [9]. Once cardiac decompensation occurs, there is a
high risk of death unless chelation therapy is dramatically intensified
[14-19]. Values <10 ms indicate high levels of cardiac iron and high risk
of cardiac decompensation [8]. It is recommended that MRI T2* should be
repeated every 2 years if T2* >20ms, every year if between 20-10 ms, every
6 months if <10 ms and even earlier if evidence of cardiac dysfunction is
documented. In a population who have not received any form of iron
chelation, this evaluation may be necessary even in younger patients
[20,21].
This was a heterogeneous population with
different chelation regimes and with problems in compliance with chelation,
hence we cannot extrapolate these results to all thalassemic patients in
India. Moreover, the study had a small sample size in which iron overload
was not compared with different chelation regimes.
In conclusion, we have demonstrated that cardiac
siderosis is present in a high proportion of patients and that this can
occur at a very early age (even below 10 years). In spite of significant
cardiac iron deposition, cardiac function in this cohort was relatively
well maintained. Serum ferritin and liver iron did not correlate with the
severity of cardiac iron overload. These findings have important
implications for the monitoring and routine management of thalassemia
patients.
Contributors:All authors were equally involved in
all aspects of study conduct and manuscript preparation.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Gradient echo T2* MR provides a rapid,
noninvasive, reproducible means for assessing myocardial iron.
What This Study Adds?
• Thalassemia patients had significantly higher cardiac iron
stores as compared to controls, and cardias siderosis was found at
an early age in poorly chelated patients.
|
References
1. Anderson LJ, Holden S, Davis B, Prescott E, Charrier
C C, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic
resonance for the early diagnosis of myocardial iron overload. Eur Heart
J. 2001;22:2171-9.
2. Tanner MA, Galanello R, Dessi C, Westwood MA, Smith
GC, Nair SV, et al. Myocardial iron loading in patients with
thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson.
2006;543-7.
3. Hershko C, Link G, Cabantchik I. Pathophysiology of
iron overload. Ann NY Acad Sci.1998;850:191-201.
4. Westwood MA, Anderson LJ, Maceira AM, Shah FT,
Prescott E, Porter JB, et al. Normalized left ventricular volumes
and function in thalassemia major patients with normal myocardial iron. J
Magn Reson Imaging. 2007;25: 1147-51.
5. Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu
PP, McGee A, et al. Survival in medically treated patients with
homozygous beta-thalassemia. N Engl J Med.1994; 331:574-8.
6. Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson
MD, Coates TD, et al. Cardiac iron determines cardiac T2*, T2, and
T1 in the gerbil model of iron cardiomyopathy. Circulation.
2005;112:535-43.
7. Carpenter JC, He Taigang, Kirk P, Anderson LJ,
Porter JB, Wood J, et al. Calibration of myocardial iron
concentration against T2-star Cardiovascular Magnetic Resonance. J
Cardiovasc Magnet Resonan.2009:11:224.
8. Kirk P, Roughton M, Porter JB, Walker JM, Tanner
MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction
of cardiac complications in thalassemia major.
Circulation.2009:120:1961-8.
9. Buja LM, Roberts WC. Iron in the heart. Etiology and
clinical significance. Am J Med.1971;51:209-21.
10. Barosi G, Arbustini E, Gavazzi A, Grasso M, Pucci
A. Myocardial iron grading by endomyocardial biopsy. A clinico-pathologic
study on iron overloaded patients. Eur J Haematol.1989;42:382-8.
11. Olson LJ, Edwards WD, Holmes Jr DR, Miller Jr FA,
Nordstrom LA, Baldus WP. Endomyocardial biopsy in hemochromatosis:
clinicopathologic correlates in six cases. J Am Coll
Cardiol.1989;13:116-20.
12. Fitchett DH, Coltart DJ, Littler WA, Leyland MJ,
Trueman T, Gozzard DI, et al. Cardiac involvement in secondary
haemochromatosis: a catheter biopsy study and analysis of myocardium.
Cardiovasc Res.1980;14:719-24.
13. Chirnomas SD, Geukes-Foppen M, Barry K, Braunstein
J, Kalish LA, Neufeld EJ, et al. Practical implications of liver
and heart iron load assessment by T2*-MRI in children and adults with
transfusion-dependent anemias. Am J Hematol. 2008;83:781-3.
14. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia
major in the UK: data from the UK Thalassaemia Register.
Lancet.2000;355:2051-2.
15. Lerner N, Blei F, Bierman F, Johnson L, Piomelli S.
Chelation therapy and cardiac status in older patients with thalassemia
major. Am J Pediatr Hematol Oncol.1990;12: 56-60.
16. Aldouri MA, Wonke B, Hoffbrand AV, Flynn DM, Ward
SE, Agnew JE et al. High incidence of cardiomyopathy in beta-thalassaemia
patients receiving regular transfusion and iron chelation: reversal by
intensified chelation. Acta Hematol.1990;84:113-7.
17. Wacker P, Halperin DS, Balmer-Ruedin D, Oberhansli
I, Wyss M. Regression of cardiac insufficiency after ambulatory
intravenous deferoxamine in thalassemia major. Chest. 1993;103:1276-8.
18. Davis BA, Porter JB. Long-term outcome of
continuous 24-hour deferoxamine infusion via indwelling intravenous
catheters in high-risk beta-thalassemia. Blood. 2000;95: 1229-36.
19. Zurlo MG, Stefano PD, Pignatti CB, Palma AD,
Melevendi C, Antonio Piga, et al. Survival and causes of death in
thalassemia major. Lancet.1989;334: 27-30.
20. Wood JC, Origga R, Agus A, Mattta G, Coates TD,
Galanello R. Onset of cardiac iron loading in pediatric patients with
thalassemia major. Hematologica.2008;93: 917-20.
21. Daar S, Pathare AV, Jain R, Zadjali SA, Pennel DJ.
T2* cardiovascular magnetic resonance in the management of thalassemia
patients in Oman. Haematologica.2009;94: 140-1.
|
|
|
 |
|

