|
|
|
Indian Pediatr 2009;46: 801-803 |
 |
Cardiogenic Shock With Hypereosinophilic
Syndrome |
|
Vinitha Prasad, L Rajam and Ashwin Borade
From the Department of Pediatrics, Amrita Institute of
Medical Sciences and Research Centre, Kochi, Kerala, India
Correspondence to: Dr Vinitha Prasad, Professor of
Pediatrics, Amrita Institute of Medical Sciences and Research Centre,
Elamakkara (PO), Kochi, Kerala 682 026, India.
Email:
[email protected]
Manuscript received: April 25, 2008;
Initial review : May 22, 2008;
Accepted: September 22, 2008.
|
|
Abstract
We report a child with hypereosinophilic syndrome who
presented with cardiogenic shock. In addition, she had skin and joint
involvement. The clinical condition improved and eosinophil counts
normalized with steroid therapy. However, the skin lesions and
hypereosinophilia relapsed on stopping the steroids. The child was
subsequently maintained in remission on low dose prednisolone.
Keywords: Cardiogenic shock, Child, Hypereosinophilic syndrome.
|
|
Hypereosinophilic
syndrome (HES) is a rare disorder characterized by unexplained, persistent
eosinophilia and end organ damage due to eosinophilic infiltration. We
report a girl with HES who presented acutely with cardiogenic shock along
with skin and joint involvement.
Case Report
A 12-year old girl was admitted with fever, swelling of
knee and wrist joints for 2 weeks and skin rashes for 6 months. There was
no history suggestive of food or drug allergy, worm infestation, throat
pain, oliguria or dyspnea.
On examination, her pulse rate was 136/min, respiratory
rate 34/min and blood pressure 84/60 mm Hg. She had raised jugular venous
pressure, bilateral pedal edema and arthritis of bilateral knee and wrist
joints. There were erythematous papules with scaling on the extremities
and angioedema of the perioral and periorbital regions. There was no
pallor, icterus, cyanosis or clubbing. The anthropometry was normal.
Examination of the chest revealed bilaterally decreased air-entry in the
infrascapular areas. Cardiac apex was at the left 5 th
intercostal space in the mid clavicular line. Heart sounds were muffled.
There were no murmurs. She had tender hepatomegaly 4 cm below the right
costal margin (span 11 cm). Spleen was just palpable. Rest of the
systemic examination was normal.
Investigations revealed hemoglobin 12.4 g/dL, total
leucocyte count of 29,000/mm 3
(polymorphs 68.9%, lymphocytes 6%, eosinophils 22.2%, absolute eosinophil
count: 6438/mm3), platelet count 647 × 103/mm3 and erythrocyte
sedimentation rate of 8 mm in first hour. Peripheral blood smear showed
marked eosinophilia, few eosinophils showed multilobation of nuclei and
hypogranulation (Fig. 1). Other cells had normal morphology.
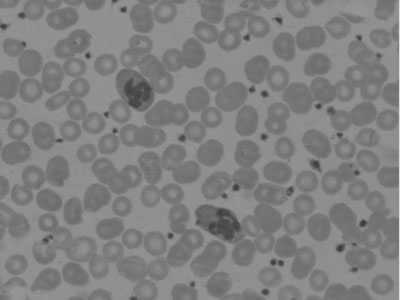 |
|
Fig.1 Peripheral smear showing eosinophils
with multilobation of nuclei and hypogranularity (Leishman stain X
100). |
Chest X-ray revealed mild pericardial effusion
and bilateral obliteration of costophrenic angles. ECG showed tachycardia
with low voltages. Echocardiogram on the second hospital day revealed a
small pericardial effusion, more over the right atrium (width 1.5 cm) and
a mild increase in the echogenicity of the myocardium. There was no
intracardiac thrombus, right ventricular dilatation, tricuspid
regurgitation or pulmonary artery hypertension. Her pulmonary artery
pressure was normal (mean pressure: 11mm Hg). Ultrasound abdomen showed
hepatomegaly, mild splenomegaly with ascites and bilateral mild pleural
effusion. Liver and renal function tests, PT and APTT were
normal.
Mantoux test, smear for malarial parasite,
microfilariae and stool examination for ova and parasites were negative.
ASO titer, Rheumatoid factor, ANA and anti-ds DNA were negative.
IgE levels were 400 IU/mL. Bone marrow aspirate showed
normocellular marrow with diffuse eosinophilia. Karyotype was normal. The
patient initially required ionotropic supports to stabilize the blood
pressure. Subsequently, a cardiac catheterization and endomyocardial
biopsy were performed. A right ventricular endomyocardial biopsy showed
hypertrophied myocardial fibres, eosinophilic infiltrates and moderate
interstitial fibrosis. Skin biopsy revealed dermal infiltration with
lymphocytes, plasma cells and few eosinophils.
Child was treated with oral prednisolone (1 mg/kg/day)
for 2 weeks. Thereafter, the steroids were gradually tapered and stopped
over 8 weeks. At 8 weeks, the WBC count was 12000/mm 3
with 0.2% eosinophils. The pericardial effusion resolved after a week. One
month after stopping the steroids, she developed fever, erythema and
angioedema over the face. The absolute eosinophil count was 2470
cells/mm3. Echocardiography was normal. She was started on prednisolone 1
mg/kg. Her skin lesions disappeared and eosinophil counts became normal
after 4weeks. She has been maintained on low dose alternate day
prednisolone (0.25 mg/kg) and is asymptomatic without peripheral
eosinophilia.
Discussion
Hyperosinophilic syndrome (HES) is a rare disease in
children, the exact prevalence is not known(1). The diagnostic criteria
for "idiopathic HES" proposed in 1975 are blood eosinophilia > 1500
cells/mm 3 for at least 6 months,
absence of an underlying cause of eosinophilia despite extensive
evaluation, and presence of end organ damage or dysfunction due to
eosinophil infiltration(2). Heart, skin and nervous system are most
frequently involved. Eosinophils release many cytotoxic substances like
eosinophil-derived neurotoxin, eosinophil cationic protein, major basic
protein, reactive oxygen species and proinflammatory cytokines, producing
end organ damage and fibrosis(2). HES must be distinguished from
eosinophilic leukemia, which is characterized by increased blood and bone
marrow blasts, eosinophilic clonality and specific cytogenetic
abnormalities(2).
Recently three subtypes of HES have been identified(3).
The myeloproliferative variant (m-HES) is characterized by an interstitial
deletion in chromosome 4q12 in cells of the myeloid lineage and
FIP1L1-PDGFRA gene fusion. Supportive evidence of m-HES includes
splenomegaly, anemia, throm-bocytopenia, increased circulating myeloid
pre-cursors, dysplastic eosinophils, elevated serum vitamin B12 and
altered leucocyte alkaline phosphatase levels. Leukemic transformation can
occur. The lymphoproliferative variant (l-HES) is associated with clonal
proliferation of phenotypically abnormal T cells. Idiopathic HES is a
subtype of the disease that is not associated with a specific chromosomal
or clonal abnormality. Our patient had overlapping characteristics of
myeloproliferative and lympho-proliferative variants.
A combination of eosinophilic myocarditis and
pericarditis with effusion contributed to the heart failure and shock in
our patient. Pulmonary embolism was ruled out. Biopsy showed myocardial
hypertrophy and moderate fibrosis, but repeat echocardiography did not
reveal any restrictive cardiomyopathy. It is possible that significant
ventricular thickening had not yet occurred, which could be detected by
routine echocardiography.
Our patient had no previous documented
hypereosinophilia and did not fulfill the classic duration criteria. She
presented with marked eosinophilia and serious cardiac involvement, which
required immediate treatment(2,4).
Corticosteroids are the initial treatment, except in
FIP1L1-PGDFRA gene fusion causing mutation. Hydroxyurea, interferon alpha,
anti IL-5 antibody mepolizumab, imatinib mesylate and bone marrow
transplantation are other therapeutic options. Anticoagulants are required
for patients with intracardiac thrombosis(5,6).
Acknowledgment
The authors acknowledge Dr Edwin Francis MD, DM,
Associate Professor, Department of Pediatric Cardiology, Amrita Institute
of Medical Sciences, for performing the cardiac catheterisation and
myocardial biopsy of our patient.
Contributors: VP and AB were involved in the
management of the patient, drafting the manuscript and review of
literature. LR was involved in the management of the patient and will act
as a guarantor for the paper.
Funding: None.
Competing interest: None stated.
References
1. Katz HT, Haque SJ, Hsieh FH. Pediatric
hypereosinophilic syndrome (HES) differs from adult HES. J Pediatr 2005;
146: 134-136.
2. Roufosse F, Cogan E, Goldman M. Recent advances in
the pathogenesis and management of hypereosinophilic syndromes. Allergy
2004; 59: 673-689.
3. Wilkins HJ, Crane MM, Copeland K, Williams WV.
Hypereosinophilic syndrome: an update. Am J Hematol 2005; 80:148-157.
4. Weller PF, Bubley GJ. The idiopathic
hypereosinophilic syndrome. Blood 1994; 83: 2759-2779.
5. Ogbogu P, Rosing DR, Horne MK. Cardiovascular
manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North
Am 2007; 27: 457-475.
6. Gotlib J, Cools J, Malone JM, Schrier SL, Gilliland
DG, Coutre SE. The FIP1L1-PDGFRA fusion tyrosine kinase in
hypereosinophilic syndrome and chronic eosinophilic leukemia: implications
for diagnosis, classification and management. Blood 2004; 103: 2879-2891.
|
|
|
 |
|

