|
|
|
Indian Pediatr 2009;46: 767-773 |
 |
Prolonged Dexmedetomidine Infusions in
Critically Ill Infants and Children |
|
Pamela D Reiter, Molli Pietras and *Emily L
Dobyns
From the Department of Pharmacy, Center for Pediatric
Medicine, and *Pediatric Intensive Care Unit,
Section of Critical Care Medicine, The Children’s Hospital, 13123 East
16th Ave, Denver, USA.
Correspondence to: Pamela D Reiter, Department of
Pharmacy, Center for Pediatric Medicine, The Children's Hospital,
13123 East 16th Ave, Denver, USA.
E-mail: [email protected]
Manuscript received: July 21, 2008;
Initial review: August 27, 2008;
Accepted: October 4, 2008.
|
|
Abstract
Objective: To present our institutional
experience with prolonged dexmedetomidine (DEX) infusions in critically
ill infants and children.
Design: Retrospective medical chart review
between January 1, 2007 and December 1, 2007.
Setting: Tertiary care pediatric teaching
hospital.
Participants: Infants and children (up to 18
years of age) who received DEX for a duration greater than 24 hours.
Main Outcome Measures: DEX dosing schema and
rationale for use. Indices describing DEX efficacy and tolerability
including change in patient-specific sedation scores, change in blood
pressure and heart rate, and change in conventional analgesia and
sedation requirements.
Results: Twenty-nine patients (age 5.32 ± 6.1 y)
were evaluated. DEX therapy was initiated at 0.36 ± 0.16 mcg/kg/hour.
One-third of patients received a loading dose (0.5-1 mcg/kg) prior to
the start of the infusion. Duration of DEX therapy was 110 ± 83 hours
(range 32-378 hours; median 76 hours). Rationale for adding DEX to
sedation regimens included: intent to extubate (n=12), intent to
reduce benzodiazepine and opioid use (n=10), exclusive continuous
sedation (n=5) and management of drug with-drawal (n=2).
Sedation scores remained stable during DEX therapy. Use of conventional
analgesia and sedation was generally reduced while receiving DEX.
Initiation of therapy was associated with a transient, yet statistically
significant reduction in HR (from 120 ± 28 bpm to 107 ± 27 bpm) (P
= 0.002), but without a change in blood pressure.
Conclusions: Prolonged DEX infusions were
associated with a reduction in concomitant analgesia and sedation
medications. DEX was well tolerated with the exception of heart rate,
which decreased during the initiation of therapy but may not represent a
clinically significant reduction.
Key words: Children, Dexmedetomidine, Sedation.
|
|
D
exmedetomidine
HCl (DEX; Precedex, Hospira Inc, Lake Forest Ill, USA) is a potent
alpha-2-adrenergic agonist that imparts sedative, analgesic and anxiolytic
effects without causing respiratory depression. DEX may be helpful in
reducing traditional sedative/analgesic use while still allowing for a
calm, comfortable, and cooperative state. The pharmacologic effect of DEX
is mediated through all four known subtypes of alpha-2 adrenergic
receptors (a2A,
a2B,
a2C,
a2D)(1)
and currently is FDA approved as a sedative for short-term use (periods
not exceeding 24 hours in duration) in adults undergoing mechanical
ventilation. Based on efficacy in adults, DEX is now being considered in
children. Pediatric experience with DEX has been predominately in the form
of case series and small reports and has focused mainly on short term or
procedural use(2-10). Little data is available describing extended
infusions in children (11-13).
We report our experience with prolonged (greater than
24 hours) use of DEX in critically ill children and attempt to
characterize indications, dosing schema, use of as needed sedation,
hemodynamic effects and clinical sedation scores associated with this
therapy.
Methods
This was a retrospective chart review of all infants
and children (up to 18 years of age) who received DEX for greater than 24
hours in duration between January 1, 2007 and December 1, 2007. Patients
were identified from the pharmacy database (Epic Hyperspace (Epic Rx)
Systems Cooperation®) at The Children’s Hospital (TCH), Denver, Colorado.
This study protocol was reviewed and approved by the Colorado Multiple
Institutional Review Board and informed parent/subject consent was waived.
Data collection included patient demographics and
indices related to DEX efficacy and tolerability. Primary outcomes
included DEX dosing schema (initial dose, maximum dose and duration),
indication/rationale for DEX use, change in patient-specific sedations
scores, change in hemodynamic parameters (systolic and diastolic blood
pressure, and heart rate) and number of conventional as needed sedation
doses required before, during and after DEX therapy. The number of "as
needed" sedation doses required during DEX therapy was calculated by
adding the number of doses required per patient per day and then taking
the mean of that number. Adequacy of sedation was assessed using a
numerical scoring system developed and validated at the Penn State
Children’s Hospital for mechanically ventilated children(14).
This scoring system allows the medical team to designate a
patient-specific sedation goal. The bedside nurse then assigns a sedation
score based on the behavior of the ventilated child. Currently, only the
Pediatric Intensive Care (PICU) uses this numerical tool in their
mechanically ventilated patients. Sedation was scored multiple times
throughout the day and was averaged over 12-hr periods for our analysis.
The daily dose of DEX (mcg/kg/hour) was calculated by
averaging the 24-hr dosing requirement of each patient. Because DEX is
often used to aid in successful extubation, our secondary intention was to
describe data regarding mechanical ventilation requirements and attempts
at extubation. The use and titration of DEX was completely at the
discretion of the medical team and the decision to extubate was based on
assessment by the unit intensivist. Hemodynamic variables (blood pressure
[mmHg] and heart rate [beats per minute; bpm]) were documented before and
during DEX infusions. All medical record charting at TCH is electronic and
patient-specific variables from bedside monitors are downloaded hourly.
Since all subjects had continuous monitoring of hemodynamic variables, we
averaged patient-specific data every 12 hours during DEX therapy. We
elected to categorize patients based on unit location (cardiac, pediatric
or neonatal ICU) because each unit is directed by separate and distinct
medical teams, and the physio-logy and diagnoses of patients are uniquely
tied to their location, This location classification then allowed for
comparison of prescribing practices between physician groups and
comparison of efficacy and tolerability of DEX based on major underlying
disease state(s).
Data are presented as mean ± standard deviation (SD) or
percentage where appropriate. Median data are reported if significant
skewness was detected. A two-tailed, paired t-test of means was
performed to determine statistical change in hemodynamic variables. A P
value less than 0.05 was considered statistical significant for any given
measure.
Results
A total of 40 patients received DEX during the study
period. Eleven patients (n=7, PICU patients; n=4, CICU
patients) were excluded because they received DEX for less than 24 hours
duration, leaving 29 patients (n=14, PICU; n=15, CICU) for
analysis. Mean age of study population was 5.32±6.1 years with a range of
0.42-18 years. Patients in the CICU subgroup were younger than patients in
the PICU subgroup (3.2±5.6 yrs vs 7.6±5.9 yrs, respectively). There
were more males (59%) than females in the study group. Ninety-three
percent of patients were mechanically ventilated at the start of DEX
therapy (86% in PICU and 100% in CICU). The 2 patients who were
spontaneously breathing at the initiation of DEX were adolescents (13 yrs
and 15 yrs) and had a diagnosis of sepsis and Steven’s Johnson syndrome,
respectively.
The primary diagnoses of the study population were
heterogeneous, and included correction/palliation of a congenital heart
defect (n=10), respiratory failure requiring mechanical ventilation
for reasons other than pneumonia (n=9), trauma (n=3),
respiratory failure requiring mechanical ventilation for pneumonia (n=3),
sepsis (n=2) and post-operative heart transplant (n=2). The
rationale for adding DEX to sedation regimens included: intent to
transition towards extubation (n=12), intent to reduce
benzodiazepine and opioid dosing (n=10), exclusive continuous
sedation (n=5) and management of drug withdrawal (n=2).
DEX Dosing. The decision to use a loading dose
was at the discretion of the prescribing physician. While our institution
provides general dosing recommen-dations of 0.3-0.7 mcg/kg/hour as a
continuous infusion dose, the medical team was responsible for all dose
titrations. DEX therapy was initiated at a mean dose of 0.36±0.16
mcg/kg/hour (range: 0.1-0.75). Figure 1 illustrates the mean
daily DEX dose requirements (mcg/kg/hour). One-third of patients (8/29)
received a loading dose (0.5-1 mcg/kg) prior to the start of the
continuous infusion (4/14 PICU patients and 4/15 CICU patients). When
daily infusion doses were averaged, the maximum dose was 0.65±0.34
mcg/kg/hour (range: 0.2-1.5), with similar values in the PICU (0.61±0.37
mcg/kg/hr) and CICU (0.67±0.32 mcg/kg/hr). The mean duration of DEX
therapy was 110±83 hours (range: 32-378 hours; median=76 hours) and was
twice as long in the PICU patients (149±102 hrs) as compared to the CICU
patients (72.6±34.6 hrs). Overall, sixteen patients (55%) had their DEX
infusion slowly tapered downward as therapy was ending. The decision to
taper the DEX infusion was directed by the medical team and was equally
likely in the CICU and PICU subgroups. In general, the taper lasted from
1-4 days in duration and typically represented 25-50% dose reductions per
day. Of the 14 patients who received DEX therapy for longer than 72 hours,
we observed a taper in 13 (93%). When DEX was used as the exclusive
continuous sedation agent (n=5 patients), the mean dose ranged from
0.3-0.48 mcg/kg/hr and none of the patients received a loading dose. The
mean duration of DEX therapy in those patients was 89 hours (range 32-168
hrs).
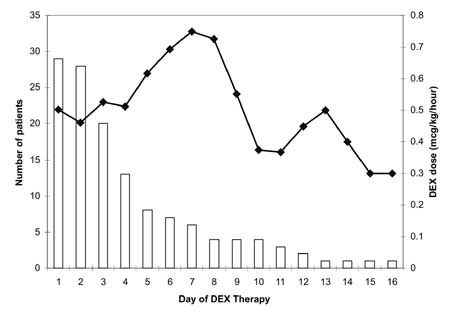 |
|
Fig.1 Mean daily dexmedetomidine (DEX)
dose (mcg/kg/hr) over time (solid line) and number of patients
receiving DEX infusions (open bars) per day. |
Sedation. The majority of patients were
receiving baseline sedation medications prior to the initiation of DEX
with a combination of continuous infusion midazolam (n=16),
intermittent midazolam or lorazepam (n=19), continuous infusion
opioid (n=22), intermittent opioid (n=20) or chloral hydrate
(n=14). During DEX therapy, sedation scores were recorded in all
twelve of the mechanically ventilated PICU patients. On average, patients
were maintained at a sedation level between 2 and 3 during the first 192
hours (8 days) of therapy and then decreased to a sedation level of 1-2
for the remaining days of therapy. This represents a trend toward more
wakeful state in those patients receiving therapy beyond 8 days.
Additional as needed doses of sedation were recorded
the day prior to starting DEX therapy, during the DEX infusion and then
again the day after DEX therapy was discontinued. Patients received a
variety of medications which included benzodiaze-pines (midazolam,
lorazepam), opioids (fentanyl, morphine, hydromorphone) and chloral
hydrate. Overall, the number of as needed doses was higher during DEX
therapy compared to before and after, and this trend remained evident in
the subgroup of patients who received DEX as exclusive continuous sedation
(Table I). Despite an increase in as needed doses,
the overall amount of sedation (continuous sedation plus as needed
sedation) was generally reduced during DEX therapy. Of the patients who
were receiving continuous opioid and BZD therapy when DEX was initiated,
54% (n=11) and 45% (n=11) were able to completely
discontinue their continuous opioid and BZD infusions during DEX therapy,
respectively. Another 18% (n=4) and 4.5% (n=1),
respectively, were able to reduce their continuous infusion requirements
by more than 50%. Six patients (4 opioid patients and 2 BZD patients)
required an increase in infusion doses even after starting DEX therapy.
Table I
Comparison of Sedation Requirements Between Study and Dexmedetomidine Groups
Sedation requirement
according to DEX
therapy |
Entire
study
(n=5) |
DEX as
exclusive continuous
sedation
(n=5) |
|
Number of needed BZD doses/day per patient |
|
1 d before initiation |
1.8±2.3 |
1.3±0.9 |
|
During DEX therapy |
2.2±1.7 |
1.9±1.3 |
|
1 d following discontinuation |
0.9±1.2 |
1.3±1.3 |
|
Number of needed opioid doses/day per patient |
|
1 d before DEX initiation |
2.2±2.4 |
1.8±2.4 |
|
During DEX therapy |
2.6±1.3 |
1.3±0.7 |
|
1 d following discontinuation |
0.9±1.4 |
1.5±3 |
|
Number of chloral hydrate doses/day per patient |
|
1 d before DEX initiation |
0.8±1 |
0.4±0.5 |
|
During DEX therapy |
1±1.24 |
1.3±1.9 |
|
1 d following discontinuation |
0.8±1.3 |
1±1.4 |
|
* All data presented as mean ± SD. Dose of sedation requirement
was calculated by adding the number of doses required per patient
per day and then taking the mean of that number; d-day. |
Hemodynamic effects. Systolic and diastolic
blood pressures, along with heart rate (HR), were documented hourly and
then averaged over 12-hour periods during DEX therapy. Overall, patients
appeared to tolerate DEX initiation well (Fig. 2). At day 4,
there appears to be a reduction in both systolic and diastolic pressures
in the CICU subgroup, but this represents the exit of older patients and
re-calibration of means to reflect a younger group of remaining subjects.
A transient, yet statistically significant decrease in HR was associated
with the first 24 hours of DEX therapy. Baseline (24 hrs prior to DEX
initiation) HR was 120±28 bpm and decreased to 107±27 bpm 24 hour post DEX
initiation (P=0.002). This association was consistent in both the
CICU and PICU subgroups. Since rapid intravenous infusion or bolus dosing
of DEX has been associated with a higher risk of hemodynamic
instability(1), we separately
analyzed those patients (n=8) who received a bolus dose of 0.5-1
mcg/kg prior to starting continuous therapy. However, we did not find the
same association. Systolic, diastolic and HR values remained stable from
12 hours prior to DEX therapy to 24 hours after DEX initiation (P=
0.56, P=0.56, P=0.78, respectively).
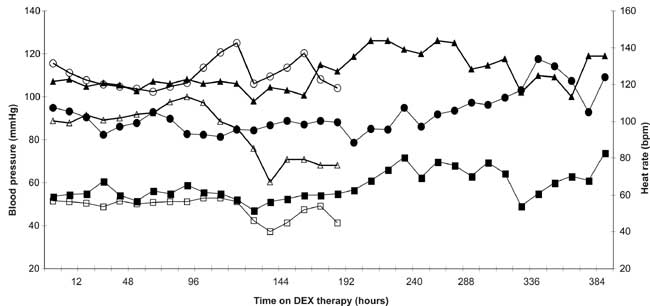 |
|
Fig. 2 Mean heart rate (circle) systolic
(triangle) and diastolic (square) blood pressure of study group
(pediatric intensive care subject = solid markers and cardiac
intensive care subjects = open markers). Each marker represents a
12-hour block of time. Dotted line represents start of DEX infusion
and first 12-hour blood pressure measurement on therapy. |
Extubation rates. Twelve patients were started
on DEX therapy considering possible extubation while 15 mechanically
ventilated patients were started on DEX for other indications. When we
excluded chronically ventilated children with tracheostomy from analysis (n=3)
and compared extubation rates between these two groups, more patients in
the intent-to-extubate group failed their first extubation attempt (30%
failure, n=3/10) compared to the rest of the study patients (7%
failure, n=1/14). These rates are comparable to our overall
extubation failure rate of 6%. Of the patients who failed their first
extubation attempt after starting DEX (n=4), 3 patients were from
the PICU and 1 was from the CICU and two of the patients had a primary
diagnosis of pulmonary hypertension. Of the 23 patients who were acutely
mechanically ventilated, 6 (26%) were extubated within 72 hours of
starting DEX, with 3 patients in the intent-to-extubate group and 3 in the
"other indications" group. Overall, patients were mechanically ventilated
1.6±8.7 day (range; 8 to 35 days) after stopping DEX therapy, which
illustrates that some patients were successfully extubated prior to the
discontinuation of DEX.
Discussion
We report the use of prolonged DEX infusions in infants
and children at doses of 0.1-1.5 mcg/kg/hour for 110±83 hours (range:
32-378 hours). Overall, concomitant opioid and benzodiaze-pine therapy was
reduced and DEX was generally well tolerated. In most patients (41%), DEX
was initiated as an adjunctive to conventional sedation in patients close
to extubation in an effort to minimize the risk of respiratory depression.
In 34% of patients, DEX was initiated with the intent to reduce/spare
benzodiazepine and opioid requirements. Overall, the use of as needed
sedation actually increased during DEX therapy, but this was most likely
due to the fact that the majority of patients were able to completely stop
or substantially reduce their concomitant opioid and BZD infusions during
this same period. Thus, the total amount of opioid and BZD could be
reduced during DEX therapy. Nevertheless, there was a small subgroup of
children in the present study that required an increase in overall
sedation, despite the addition of DEX.
Changes in blood pressure and HR (bradycardia) have
previously been reported in patients receiving DEX, especially when a
loading dose is pres-cribed(3,15-17). DEX initiation was not associated
with any significant change in blood pressure. However, DEX was associated
with a statistically significant reduction in HR, with a mean HR reduction
of 13 bpm from baseline (24 hours prior to DEX) to 24 hours after the
initiation of DEX. This drop in HR may represent an important hemo-dynamic
effect of DEX or may correspond to improved sedation with less agitation.
Arguably, a mean HR reduction of 13 bpm may not denote a clinically
significant decrease. We did not observe the same impact on HR in the
small group of patients (n=8) who received a DEX loading dose prior
to the initiation of a continuous infusion. One plausible explanation for
this lack of effect may be selection bias. It is possible that those
patients who received a DEX bolus were deemed more hemodynamically stable
by the medical team and hence judged as better candidates for a bolus,
compared to the rest of the study group.
DEX is an appealing agent to use in patients close to
extubation because of its relative lack of respiratory drive depression.
Therefore, we analyzed the association of extubation failure/success and
the rational of DEX initiation. We observed a higher extubation failure
rate (30%) in those patients specifically started on DEX with the intent
to extubate, compared to the rest of the study group requiring mechanical
ventilation (7% failure rate). A possible explanation for the higher
failure rate in the intent-to extubate group may be due to the higher
proportion of PICU patients in that group (60%) compared to the rest of
the study group (50%). The PICU patient population is generally more
diverse in terms of pulmonary pathology while the CICU group tends to be
more homogenous and extubates quickly during the post-operative period.
Additionally, the CICU physician group uses a ventilator weaning protocol,
unlike the PICU physician group. This weaning protocol may aid in
assessing patient readiness for extubation. However, one can not overlook
the possibility that the use of DEX may change the way a child exhibits
their readiness to extubate. It is possible that a child may appear alert
and cooperative, yet may in fact be too sedated for a successful
extubation. As only 4 patients had documented sedation scores in the
planned extubation group, we are unable to relate sedation score to
success of failure of extubation.
There were limitations to our study that are attendant
to any retrospective review. Since this was an observational analysis, we
were not able to control for parameters that may have impacted outcomes.
In particular, the use of DEX was completely at the discretion of the
intensivist – including the decision to use a loading dose and all dosing
titration maneuvers. Additionally, this was an open label study and did
not include a control group, therefore we can report only associations of
DEX with outcomes and can not assume any causality. Furthermore, the
decision to use additional sedation medications as well as the readiness
for extubation was at the judgment of the medical team. While DEX offers
the clinician another choice for continuous and titratratable sedation in
the ICU, it does not seem to be the universal solution for all children
requiring sedation and analgesia. In an effort to describe the best
candidate for DEX, controlled prospective and blinded trials must be
performed.
Contributors: PDR was responsible for the study
idea, design and data analysis. MP collected and co-analyzed all data and
reviewed the manuscript. ELD reviewed and edited the manuscript; provided
key insight to ICU management issues; contributed important data
interpretation and intellectual content. All authors approved the final
content of the manuscript.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Dexmedetomidine has short-term efficacy and
tolerability in infants and children and has sedative, analgesic and
anxiolytic effects, without causing respiratory depression.
What This Study Adds?
• Dexmedetomidine infusions in infants and
children for 110 ± 83 hours were associated with an overall
reduction in concomitant opioid and benzodiazepine therapy but
associated with fall in heart rate during the first 24 hour of
therapy.
|
References
1. Scholz J, Tonner PH. Alpha-adrenoceptor agonists in
anaesthesia: a new paradigm. Curr Opin Anaesthesiol 2000; 13: 437-442.
2. Tobias JD, Berkenbosh JW. Initial experience with
dexmedetomidine in paediatric-aged patients. Paediatr Anaesth 2002; 12:
171-175.
3. Tobias JD, Berkenbosh JW, Russo P. Additional
experience with dexmedetomidine in pediatric patients. South Med J 2003;
96: 871-875.
4. Tobias JD, Berkenbosh JW. Sedation during mechanical
ventilation in infants and children: dexmedetomidine versus midazolam.
South Med J 2004; 97: 451-455.
5. Berkenbosh JW, Wankum PC, Tobias JD. Prospective
evaluation of dexmedetomidine for non-invasive procedural sedation in
children. Pediatr Crit Care Med 2005; 6: 435-439.
6. Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI,
Ersoy OM. Sedative, hemodynamic and respiratory effects of dexmedetomidine
and propofol in children undergoing magnetic resonance imaging
examination. Anesth Analg 2006;103: 63-67.
7. Kalyanaraman M, Costello JL, Starr JP. Use of
dexmedetomidine in patients with trisomy 21 after cardiac surgery. Pediatr
Cardiol 2007; 28: 396-399.
8. Ard J, Doyle W, Bekker A. Awake craniotomy with
dexmedetomidine in pediatric patients. J Neurosurg Anesthiol 2003; 1593:
263-266.
9. Walker J, Maccallum M, Fisher C , Kopcha R, Saylors
R, McCall J. Sedation using dexmedetomidine in pediatric burn patients.
[abstract]. J Burn Care Res 2006; 27: 206-210.
10. Shukry M, Ramadhyani U. Dexmedetomidine as the
primary sedative agent for brain radiation therapy in a 21-month old
child. Pediatr Anesth 2005; 15: 241-242.
11. Buck ML, Willson DF. Use of dexmedetomidine in the
pediatric intensive care unit. Pharmacotherapy 2008; 28: 51-57.
12. Kiski C, Hosokawa K. Dexmedetomidine in fast-track
pediatric cardiac surgery.[abstract] Crit Care Med 2007; 35 (12 Suppl):
A343.
13. Rapan KA, Lewin JJ, Lee CK, Veltri MA, Easley RB.
Use of dexmedetomidine in a pediatric intensive care unit. [abstract] Crit
Care Med 2007; 35 (12 Suppl): A876.
14. Popernack ML, Thomas NJ, Lucking SE. Decreasing
unplanned extubations: utilization of the Penn State Children’s Hospital
Sedation Algorithm. Pediatr Crit Care 2004; 5: 58-62.
15. Talke P, Richardson CA, Scheinin M, Fisher DM.
Postoperative pharmacokinetics and sympatholytic effects of
dexmedetomidine. Anesth Analg 1997; 85: 1136-1142.
16. Bloor BC, Ward DS, Belleville JP, Maze M. Effects
of intravenous dexmedetomidine in humans: II. Hemodynamic changes.
Anesthesiology 1992; 77: 1134-1142.
17. Gerlach AT, Dasta J, Armen S, Smith J, Steinberg S,
Martin L, et al. Titration protocol reduces hypotension during
dexmedetomidine infusion in critically ill surgical patients. [abstract]
Crit Care Med 2006; 34(suppl): A148.
|
|
|
 |
|

