|
Bacterial Profile of Sepsis in
a Neonatal Unit in South India |
Kurien Anil Kuruvilla, Swati Pillai, Mary Jesudason* and Atanu Kumar Jana
From
the Department of Neonatology and Microbiology*, Christian Medical College Hospital,
Vellore
632 004, Tamil Nadu, India.
Reprint requests: A.K. Jana, Professor and Head, Neonatology Department, Christian Medical College Hospital, Vellore
632 004, Tamil Nadu, India.
Manuscript received: November 26, 1997; Initial review completed: January 14, 1998;
Revision accepted: February 9, 1998.
Abstract:
Objective: To study the pattern of sepsis in a neonatal unit in south India and assess the influence of maternal factors on early onset sepsis
(EOS).
Design: Prospective survey from
1995-
1996. Setting: Medical College Hospital. Subjects: All inborn babies who had clinical signs of sepsis or were born to mothers with potential risk factors for infection were screened for sepsis. Neonatal septicemia was defined as a disease of infants who were younger than 1
month of age,
were clinically ill, and had positive blood cultures. Results: Among 13367
live
births in the
study period, there were
131 episodes of neonatal septicemia among 125 newborn infants, 18 (14.4%) of whom died. Thirty (24%) had EOS (≤ 48 hours) and 95 (76%) had late onset sepsis (LOS) (≥ 48 hours). Sepsis occurred in 9.8 per 1000 livebirths and 4.4% of all nursery admissions. E. coli and E. fecalis were the predominant organisms causing E05, while Klebsiella and E. fecalis were the predominant organisms in LOS. The mean gestational age (GA) and birth weight (BW) of babies with EOS was significantly higher than those with LOS. Maternal factors significantly associated with EOS were meconium staining of liquor and multiple vaginal examinations. Conclusions: The incidence of neonatal bacterial sepsis is 9.8 per 1000 livebirths. E. coli-and Klebsiella were the most common organisms causing EOS and LOS, respectively: E. fecalis was also a major pathogen, both in EOS and LOS.
Key words:
Newborn, Sepsis.
SEPTICEMIA is a major cause of morbidity and mortality in the
newborn. The bacteriological profile of neonatal sepsis is
constantly under change with advances in the early diagnosis and treatment of sepsis. and the increased survival of tiny preterm babies.
Thus a rational protocol for sepsis management must be based on
adequate knowledge of the causative organisms and their antimicrobial sensitivity pattern.
In developed countries, Group B Streptococcus (GBS) and Coagulase
Negative Staphylococci (CONS) are the most common etiological agents for early onset sepsis (EOS) and late onset sepsis (LOS), respectively. However, in the developing nations these organisms are rare, with an entirely different bacterial spectrum. This study conducted in south India aimed to profile over two years the bacteriological spectrum of neonatal septicemia in a large perinatal center providing neonatal intensive. care facilities.
Subjects and Methods
The study was conducted prospectively
in babies born in the Christian Medical
College Hospital (CMCH), Vellore, from January 1st, 1995 to December 31st, 1996. This is a tertiary care center in a semi-urban area in South India. Antenatal, perinatal
and obstetric data was obtained for all 13367 births in the
hospital in this period, of which 9678 (72.4%) cases were
booked. The majority of patients were in the middle and lower
middle socio-economic class. The nursery has 12 intensive care
cots including three ventilator beds.
Infants with clinical signs of sepsis or those who were born to mothers with potential risk factors for infection were screened for sepsis. Risk factors in the mother for EOS included prolonged rupture of membranes (PROM >24 hours), maternal pyrexia, untreated urinary tract infection (UTI), chorioamnionitis, or multiple vaginal examinations (>3). Blood cultures were collected from a peripheral vein only after cleaning the skin with iodine and alcohol. The blood was inoculated into two blood culture bottles containing Brain Heart Infusion (BHI) broth and Biphasic MacConkey Medium (BPMM)(1) using a fresh sterile needle. The inoculated bottles were incubated at 3TC, the BHI in 5-10% carbon dioxide atmosphere and the BPMM aerobically. Subcultures were taken from BHI after 24 hours, 7 days and 14 days. The BPMM medium was examined daily for growth. Organisms were identified as per standard procedures(2).
Neonatal septicemia was defined as a disease of infants who were younger than 1 month of age, were clinically ill, and had positive blood cultures(3). Cases in which blood culture was positive and the specimen obtained within 48 hours of birth were considered as EOS, and LOS if obtained after 48 hours(4). Blood total and differential counts, CSF analysis and chest radiographs were done in all cases. Intravenous crystal- line penicillin and gentamicin were the first
line of antibiotics administered for babies at risk of EOS, while in LOS cefotaxime and
amikacin were administered initially. Anti-biotic therapy was continued based on the isolation of organisms in the blood culture and its sensitivity pattern. Parenteral fluids and nutrition, blood transfusions, inotropic support, and ventilatory care were provided if necessary. We did not use intravenous immunoglobulin (IVIG).
Data was analyzed according to gestational age, birth weight, postnatal age, and
presence of antenatal risk factors. Infants with EOS and LOS were compared using t-test for continuous variables, and by chi-square for discrete variables. A p-value of < 0.05 was taken as being statistically significant.
Results
During the 2 year study period, 2967 (22.2%) of the 13367 babies born in CMCH were admitted to the nursery. There were 1314 (9.8%) preterm births. One hundred and twenty five babies had 131 episodes of sepsis, including 30 (22.9%) infants with EOS and 95(77.1%) babies with 101 episodes of LOS. The incidence of sepsis was 9.8 per 1000 live births and sepsis accounted for 4.4% of all nursery admissions.
The. mean (SO) GA of infected babies was 36.1(±
3.5) weeks and mean BW was 2280 (±
805) g. Eighty five (68.3%) were male infants. Mortality due to
sepsis was 18 (14.4%), representing 19.1% of all deaths during the study period.
Distribution of Organisms
E. coli (n
=
7) and E. fecalis (n
=
6) were the most common organisms causing EOS (Fig. 1). Group A and Group B Streptococci (GAS, GBS) contributed to 2 cases each in EOS.
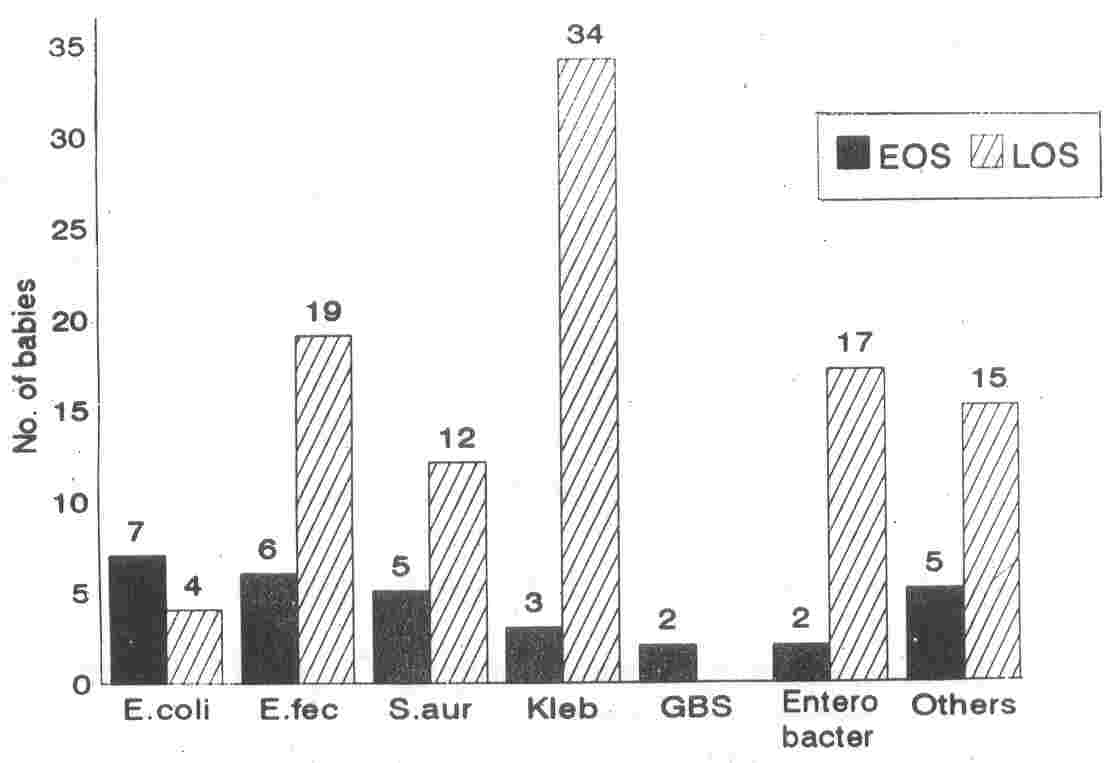 |
|
Fig. 1. Organisms
analyzed
by time
of
onset
of
sepsis.
EOS: early onset sepsis; LOS: late enset sepsis;
E. fec: Enterococcus fecalis; S.
aur: Staphylococcus aureus; Kleb: Klebsiella sp.; GBS: Group B Streptococcus; Others: Coagulase
Negative Staphylococcus (n
= 7), Pseudomonas sp. (n = 4), Group A
Streptococcus
(n
= 2),
Acinetobacter
sp. (n
= 2). |
Among babies with LOS, the major
causative organism was Klebsiella (n
=
34). The other organisms frequently encountered in LOS were E.
lecalis
(n
=
19) and Enterobacter (n
=
16). Overall Gram Negative Bacilli (GNB) were the infecting organisms in 60 (59.4%) babies with LOS.
E. coli was responsible for 7 (23.3%) cases of EOS and 4 (4.2%) cases of LOS. The mean (SD) GA of the infants was 37.5 (±
3.4) weeks, mean (SO) BW was 2567 (±
710) g and mortality was 2 (18.1%) (Table I), Only three of the 37 infections due to Klebsiella were EOS. The mean. (SO) BW and GA of the infected infants was 1871 (±
767) g and 35.1 (±
3.0) weeks, respectively. Mortality was 8 (21.6%) (Table I). An equal proportion (20%) of EOS (6 cases) and LOS (19 cases) were caused by E.
lecalis. The mean BW was 2345 (±692)
g
and mean GA was 36.9 (±
3.4) weeks. One baby who died had multiresistant E. lecalis sepsis.
Analysis of distribution of organisms by birth weight revealed that Klebsiella caused 14 (48.2%) of all infections in babies
<1500 g and 23 (225%) babies weighing
≥1500 g. Other organisms causing sepsis in very low birth weight (VLBW) infants were
Enterobacter (n
=
6) and E. lecalis (n
=
5).
Both babies with GBS septicemia weighed more than 2500 g.
Early Onset Septicemia and Perinatal
Factors
Among all preterm babies born in CMCH, 7 (0.5%) developed EOS as compared to 23 (0.2%) term infants who developed EOS (p
=
0.003). The incidence of EOS in babies < 1500 g was 0.6% and 0.2% in those
≥1500 g.
The mean BW and GA for babies with EOS was significantly higher than those
with LOS (p < 0.05)
(Table
II).
Among the
various maternal risk factors for neonatal sepsis, meconium staining of amniotic fluid and multiple vaginal examinations were found to be significantly associated with
EOS (Table II).
Overall 9 (30%) mothers of babies with EOS had one or more antenatal predisposing factors for sepsis.
TABLE I
Summary of Major Groups of Infecting Organisms
|
Organism |
n |
No. of
EOS |
Mean (SD)
BW (g) |
Mean (SD)
GA (weeks) |
Mean (SD) day
of onset |
Mortality |
|
E. coli |
11 |
7 |
2476
(756)
|
37.3 (3.2)
|
2.9 (2.6) |
2 |
|
E. fecalis |
25 |
6 |
2345 (692) |
36.9 (3.4) |
5.4 (5.0) |
1 |
|
Klebsiella sp. |
37 |
3 |
1883 (743) |
35.2 (3.0) |
4.4 (2.4) |
8 |
|
Enterobacter sp. |
19 |
2 |
1813 (607) |
34.7 (4.0) |
6.8 (3.8) |
7 |
|
Staph. aureus |
17 |
5 |
2105 (628) |
35.7 (3.4) |
5.4 (3.6) |
0 |
BW: birth weight; GA: Gestational age; EOS: Early
onset sepsis
TABLE
II
Comparison of Perinatal Factors for Early and Late Onset Sepsis
|
Perinatal factors |
EOS
(n
=
30)
|
LOS
(n
=
95)
|
P
<0.00001 |
|
Mean postnatal age (SD) (days)
|
1.3±0.47 |
6.4±4.8 |
0.01 |
|
Mean BW (SD) (g) |
2600±569 |
2178± 844 |
0.009 |
|
Mean GA (SD) (weeks) |
37.6±3.0 |
35.6±3.5 |
ns |
|
Male sex |
21 (70) |
65 (68.4) |
|
|
Maternal risk factors |
|
|
|
|
PROM >24 h |
4 (13.3) |
12 (12.6) |
ns |
|
Maternal pyrexia |
2 (6.6) |
1 (1.1) |
ns |
|
Chorioamnionitis |
1 (3.3) |
3 (3.2) |
ns |
|
Meconium liquor |
9 (30) |
8 (8.4) |
0.002 |
|
Multiple vaginal examinations |
6 (20.0) |
2 (2.1) |
0.0005 |
|
UTI |
1 (3.3) |
1 (1.1) |
ns |
|
Mode of delivery
|
|
|
|
|
Normal
|
16 (53.3) |
49 (42.1) |
ns |
|
Instrumental |
6 (20) |
12 (12.6) |
|
|
LSCS |
8 (26.7) |
43 (45.2) |
|
|
Deaths |
5 (16.7) |
13 (13.6) |
ns |
Figures in parentheses indicate percentages. BW: birth weight;
GA: gestational age; PROM: pro-longed rupture of membranes;
UTI: urinary tract infections; LSCS: lower segment Caesarean section;
ns: not significant.
Meningitis:
Only 3 babies had bacterial
meningitis; 2 had EOS due to Klebsiella and E. fecallis, and one had LOS due to E. fecalis diagnosed on day 4. None of these babies died.
Pneumonia: There were 10 (8%) patients with clinical and radiographic evidence of pneumonia, all with EOS. Three episodes were caused by E. coli, and 2 each by Klebsiella and E. fecalis.
Notable Features of Klebsiella Septicemia
Among the babies with Klebsiella septicemia, 9 (24:3%) babies had multiple abscesses, 8 (21.6%) had conjugated hyper-bilirubinemia, and 3 (8.1 %) had septic arthritis of the hip joint.
Antibiogram: The
antibiotic sensitivity pattern shows that
E. coli
was usually sensitive to cefotaxime, gentamicin, and amikacin, and E. fecalis was sensitive to penicillin, cefotaxime and amikacin
(Table
III).
However, Klebsiella and Enterobacter responsible for most LOS were resistant to the aminoglycosides and cephalosporins, and sensitive only to ciprofloxacin and imipenem.
Outcome: There were 18 (14.4%) sepsis-related deaths, 5 due to EOS and 13 due to LOS. Two infants with EOS who died had
E.
coli
septicemia. In LOS the common
organisms responsible for mortality were
Klebsiella(n
=
7) and
Enterobacter
(n
=
5).
Discussion
This study from one of the largest perinatal centers in India reports on the incidence of neonatal sepsis, its mortality and the risk factors contributing to early neonatal sepsis.. In this study, the incidence of neonatal sepsis and meningitis was 9.8 and 0.2 per 1000 livebirths, respectively. This is comparable to previously published literature which show an incidence of 1 to 12.4/ 1000 livebirths(5,6) in neonatal sepsis, and 0:3 to 2.8/1000 livebirths in neonatal men- ingitis(7,8). However other centers in India found upto 34/1000 livebirths having neo- natal sepsis(9).
The mortality rate of 16.7% and 13.6% in EOS and LOS, respectively in this study is lower than in manystudies(10,ll). The percentage of babies with meningitis is also very small. Adequate anticipation, early diagnosis and treatment with the use of
appropriate antibiotics in a perinatal center where the majority of mothers are booked patients would contribute to this low incidence of meningitis and low mortality rate.
The mean BW and GA of infected babies in this study was similar to reports from
Pakistan(12) and Dubai(13) but higher than in other
studies(14,15). This would also contribute to the lower
mortality rate. Investigators have used widely different time periods to demarcate EOS and LOS. The definition
of EOS varies from infection within the first 24-72 hours of life (13,16) upto 7 days(12,17). Many authors have not attempted to differentiate EOS and LOS(10,11). In this study, we defined EOS as sepsis occurring less than 48 hours after birth since infection in this period could be attributed to antenatal factors. LOS was defined as infections beyond 48 hours of birth which were increasingly nosocomial in origin(4). This is further corroborated by the antibiotic resistance pattern of the organisms causing EOS and LOS.
In other reports EOS contributed to 38% to 85% of neonatal sepsis(ll,13,14), unlike in this study where 22.9% of cases were EOS. The major causative organism in EOS was E. coli, similar to the findings of Placzek and Yu(15,19). Other studies from the developing world found Klebsiella as the common organism in EOS(12,13,17), in contrast to the developed nations where CBS causes upto 52% of EOS(14,16).
Klebsiella was the predominant organism causing LOS, followed by E. fecalis and Enterobacter. In developed countries, CONS is the major etiological agent for LOS(14,15). Similar to our findings, Gupta et al, reported conjugated hyperbilirubinemia and septic arthritis in babies with Klebsiella septicemia(20).
Though GBS is rare in developing countries, other Streptococci have been re- ported(10,18). We found that" 20% of EOS' and LOS were caused by E. fecalis. Two of these babies
had meningitis and one died
of me.
TABLE III
Antibiotic Resistance Pattern of Major Organisms Isolated
|
Organism |
Time of
Onset |
No.of
babies |
Ampi
|
Amik
|
Cft
|
Cip
|
Czd
|
Czl |
Gen |
lmi |
Oxa |
Pen |
Van |
|
Esch. coli
|
EOS |
7 |
1 (14) |
0 |
0 |
0 |
0 |
0 |
1 (14) |
- |
-
|
-
|
-
|
|
LOS |
4 |
2 (50) |
0 |
1 (25) |
1 (25) |
1 (25) |
1 (25) |
1 (25) |
- |
-
|
-
|
-
|
|
E. fecalis
|
EOS |
6 |
2 (33) |
0 |
1 (16) |
|
|
0 |
4 (66) |
- |
0 |
2 (33) |
0 |
|
LOS |
19 |
4 (21) |
1 (5) |
1 (5) |
- |
- |
1 (5) |
16 (84) |
|
2 (10) |
4 (21) |
0 |
|
Staph. aureaus |
EOS |
5 |
5 (100) |
- |
0 |
0 |
- |
0 |
1 (20) |
-
|
0
|
5 (100) |
0 |
|
LOS |
12 |
12 (100) |
|
4 (33) |
1 (8) |
1 (8) |
4 (33) |
5 (41) |
|
5 (41) |
12 (100) |
0 |
|
Klebsiella
|
EOS |
3 |
1 (33) |
0 |
1 (33) |
0 |
1 (33) |
1 (33) |
1 (33) |
0 |
-
|
-
|
-
|
|
LOS |
34 |
17 (50) |
26 (76) |
21 (61) |
0 |
22 (64) |
25 (73) |
29 (85) |
0 |
-
|
-
|
- |
|
Enterobacter
|
EOS |
2 |
2 (100) |
1 (50) |
1 (50) |
1 (50) |
2 (100) |
2 (100) |
2 (100) |
0 |
- |
-
|
-
|
|
LOS |
17 |
14 (82) 15 (88) |
14 (82) |
7 (41) |
13 (76). |
14 (82) |
14 (82) |
0 |
- |
- |
-
|
Figures in parenthesis inditate percentage of antibiotic resistance. EOS: early onset sepsis; LOS: late onset sepsis; Ampi: Ampicillin; Amik: Amikacin; Cft: Cefotaxime; Clip: CiprofIoxacin; Czd: Ceftazidime; Czl: Cefazolin; Gen: Gentamicin; lmi: lmipenem; Oxa: Oxacillin; Pen: Penicillin; Van: Vancomycin.
"-" indicates sensitivity not tested.
Among the various antenatal factors in the mother described to
predispose neonates to sepsis, we found only meconium staining of liquor and multiple vaginal examinations to be significantly associated
I
with EOS. Similar to the findings of the present study J Placzek(15) reported that 29.7% mothers of infants having EOS had one or more risk factors for infection.
The mean BW and GA of babies with LOS was significantly 'lower than those with EOS, as shown in previous stud- ies(14,15,17). Prolonged hospitalization and more intervention predisposes these preterm and LBW babies to hospital- acquired infections.
A distinct difference was seen in the antibiogram of the organisms causing EOS and LOS. Organisms in EOS were usually sensitive to commonly used antibiotics. However, the organisms causing LOS were multiresistant. Earlier, Bannon(21) has reported treating resistant Enterobacter septicemia with ciprofloxacin. In treating babies with LOS having multiresistant organisms, 'we have had to use ciprofloxacin, imipenepl or vancomycin, based on the culture
and sensitivity report. The outcome was good as evidenced by the
low mortality rate.
Thus, in this study, apart from E.coli
and
Klebsiella
which were the most common organisms responsible for EOS and LOS, respectively, E. fecalis was also found to be a major pathogen in
both EOS and LOS. With early diagnosis and treatment and the judicious use of appropriate antibiotics, neonatal morbidity and mortality due to sepsis can be markedly decreased.
|
1.
Koshi G, Mukundan U, Mathews M. Advantages of MacConkey biphasic medium
for blood culture.' Indian
J
Med Res 1985; 81:
584-590.
2.
MyersRM, Koshi G. Diagnostic Procedures in Medical Microbiology and Immunology
Serology. Microbiology Laboratories, Christian Medical College Hospital Vellore, 1982; p 19.
3.
Freij BJ, McCracken tHo Acute Infections. In: Neonatology: Pathophysiology and Management of the Newborn, 4th edn. Eds. Avery GB, Fletcher MA, MacDonald MG. Philadelphia, J.B. Lippincott
Company, 1994;p 1088.
4.
Peter G, Cashore WJ. Infections acquired in the nursery: Epidemiology and Control. In: Infectious Disease of the Fetus and Newborn Infant, 4th edn. Eds. Remington JS, Klein JO. Philadelphia, W.B. Saunders Company, 1995; p 1274.
5.
Horton JA, Hemming VG, Croess DF. A survey of infants with neonatal sepsis in a US army hospital. Military Med 1989; 154: 584-587.
6.
Eriksson M. Neonatal septicemia. Acta Pediatr Scand 1983; 72: 1-8.
7.
Klein JO, Marcy SM. Bacterial sepsis and meningitis. In: Infectious Diseases of the Fetus and Newborn Infant, 4th edn. Eds. Remington JS, Klein JO. Philadelphia, W.B. Saunders Company, 1995; p 847.
8.
Stoll BJ. The global impact of neonatal infection. In: Clinics in Perinatology: Infections in Perinatology. Eds. Stoll BJ, Weisman LE. Philadelphia, W.B. Saunders Company, 1997; 24: 3-4.
9.
Choudhury P, Srivastava G, Aggarwal DS, Saini L, Gupta S. Bacteriological study, of neonatal infection. Indian Pediatr 1975; 12: 459-463.
10.
Bhakoo ON, Agarwal KC, Narang A, Bhattacharjee S. Prognosis and treatment of neonatal septicemia. A clinico
bacterialogical study of 100 cases. Indian Pediatr 1974; 11: 519-28.
11.
McCracken GH, Shinefield HR. Changes in the pattern of neonatal septicemia and meningitis. Am
J
Dis Child 1966; 112: 33-39. .
12.
Bhutta ZA, Naqvi SH, Muzaffar T, Farooqui BJ. Neonatal sepsis in Pakistan. Acta Pediatr Scand 1991; 80: 596-601.
13.
Daoud AS, Abuekteish F, Obeidat A, EI-Nassir Z, AI-Rimawi H. The changing face of neonatal septicemia. Ann Trop Pediatr 1995; 15: 93-96.
14.
Sanghvi KP, Tudehope DI. Neonatal bacterial sepsis in a neonatal intensive care unit: A 5 year analysis.
J
Pediatr Child Health 1996; 32: 333-338.
15.
Placzek MM, Whitelaw A. Early and late neonatal septicemia. Arch Dis Child 1983; 58: 728-731.
16. Vesikari T, Janas M, Gronroos P,
'Tuppurainen N, Renlund M, Kero P, et al. Neonatal septicemia. Arch Dis Child 1985; 60: 542-546.
17.
Chugh K, Aggarwal BB, Kaul VK, Arya Sc. Bacteriological profile of neonatal septicemia. Indian
J
Pediatr 1988; 25: 961-
965. '
18. Chaturvedi P, Agrawal M, Narang P. Analysis of blood culture isolates, from neonates of a rural hospital. Iridian Pediatr 1988; 25: 171-178.
19.
Yu WH. Neonatal infection control policies in Australia.
J
Pediatr Child Health 1990; 26: 252-256.
20.
Gupta P, Murali MV, Faridi MMA, Kaul PB, Ramachandran VG, Talwar V. Clinical profile of Klebsiella
septicemia in neonates. Indian
J
Pediatr 1993; 60: 565-572.
21.
Bannon MJ, Stutchfield PR, Weindling AM, Damjanovic V. Ciprofloxacin in neonatal Enterobacter cloacae septicemia. Arch Dis Child 1989; 64: 1388-1391.
|
|
| |
