K.L. Srivastava, M. Bansal, S. Gupta, R. Srivastava*, R.K. Kapoor, I. Wakhlu and B.S. Srivastava*
From the Department of Pediatrics, King George's Medical College, Lucknow and Central Drug Research Institute*, Lucknow 226 003, U.P., India.
Reprint requests: Dr. K.L. Srivastava 29, Kasturba Marg, Lucknow Cantt, Luclcnow, U.P., India.
Manuscript received: April 11, 1997; Initial review completed: June 19, 1997;
Revision accepted: April 16, 1998.
Abstract:
Objective: To evaluate diagnostic potential of three immunological tests, namely, detection of
H37RV antigen of M. Tuberculosis in CSF, detection of antibodies
IgG) against
H37RV in CSF and detection of antibodies IgG) against H17Rv in serum for diagnosis of tuberculous meningitis in children. Subjects: 50 children diagnosed as patients of tuberculous meningitis were included as
cases and 48 children with CNS diseases of non tubercular etiology [pyogenic meningitis (n
= 31),
encephalitis (n = 10), seizure disorder of unknown etiology (n = 5), brain tumor (n
= 2)] served as controls. Methods: H37RV antigen of M. tuberculosis was detected in CSF by Dot ELISA, and antibodies
IgG) against HJ7RV in CSF and serum were detected by Plate ELISA. Results: Detection of H17Rv antigen in CSF was the most sensitive (90%) and specific (95.83%) with positive and negative predictive values of 95.74% and 90.19%, respectively, followed by detection of antibodies in CSF (sensitivity-74%, specificity-89.58%, positive predictive value- 88.10%, negative predictive value-76.78%). Detection of antibodies in serum had low sensitivity (50%), specificity (91.67%), positive predictive value (86.21%) and negative predictive value (63.76%). Conclusions: Detection of antigen in CSF is a rapid, sensitive and specific test for diagnosis of tuberculous meningitis in children. Detection of antibody in CSF may be useful in some cases but needs further evaluation. Detection of antibody in serum does not appear to be useful for diagnosis of tuberculous meningitis.
Key words: ELISA, Tuberculous meningitis.
TUBERCULOSIS is a major public health problem in all developing countries including India. World wide, it kills more people than any other single infectious
disease(1). Out of all forms, tuberculous meningitis (TBM) is the most devastating with
mortality ranging from 13% to 71.9%(1,2). Early diagnosis and treatment is essential to reduce mortality and long term morbidity associated with TBM. The
'Gold
Standard' for the diagnosis of any infection is bacterial isolation, and demonstration of tubercle bacilli would be the best way to confirm the tubercular etiology in meningitis, but unfortunately, the yield of tubercle bacilli on AFB staining is very low. Further, a very large number of bacilli, at least
10,000/ml is required for the bacilli to be demonstrated in smears(3) which may not be the case with every specimen. Culture
takes a long period of 6 to 8 weeks and one cannot wait for culture report to start the therapy. There is therefore an urgent need for finding alternative method of diagnosis of tuberculosis which are reliable, time saving, cost effective and easy to perform.
Attempts have been made to improve the sensitivity and speed of detection of tubercle
bacilli or their components by techniques such as radiometric
determination of bacterial growth, gas chromatography, DNA hybridization
and Polymerase chain reaction, but the involved high cost and the
required high level of technical expertise are important barriers in
their use(4). Enzyme linked immunosorbent assay (ELISA) has the advantage of being a cheaper and time saving test requiring relatively less technical expertise in performing the test. The present study was undertaken to determine the diagnostic potential of detection of
H37RV antigen of M. tuberculosis in CSF by dot ELISA, and detection of antibodies (IgG) against
H37RV antigen in CSF
and serum by plate ELISA in diagnosis of tuberculous meningitis in children.
Subjects and Methods
Subject Selection
In this case control study, conducted in Children's Ward of Gandhi Memorial and Associated Hospitals, Lucknow,
India, between May 1994 to May 1995, eligibility criteria for initial enrollment were defined as: children of either sex, below 12 years of age, presenting with features of CNS infection (fever, and altered sensorium,
and convulsions, with or without headache, vomiting and neurological deficit), who had not been treated with antitubercular
drugs in last 14 days, or antibiotics in last 7 days prior to admission.
Detailed history and findings of clinical examination at the time of
admission were noted. Written consent was obtained from the parents or
the nearest relatives of each patient. Lumbar puncture was done and CSF
obtained was subjected to biochemical and cytological examination, Gram's and Ziehl Neelson's staining, culture for mycobacterium in LJ media for 6-8 weeks, and bacterial and fungal culture. Part of CSF and serum from the blood drawn at the time of admission was stored in autoclaved eppendroff vials at -20o C for further use. Cranial computed tomography was done on each patient.
Patients were carefully followed-up for 6 months and the details of therapy given and response to therapy were noted. Patients were labeled to be suffering from tuberculous meningitis if the following criteria were met: (a) CSF findings; pleocytosis of more than 20 cells per cumm with more than 60% being lymphocytes, and CSF proteins more than 100 mg/ dl and CSF sugar less than 60% of concomitant blood sugar levels (all 3 mandatory) with or without positive smear/culture for tubercle bacilli, and (b) clinical course and response to exclusive antitubercular
treatment (gold standard for diagnosis adopted in the present study) and (c) CT scan findings (2 out of 4 following features)- exudates in basal cistern or sylvian fissure, hydrocephalus, infarct, Gyral
enhancement. All three (a, b and c) were required to make a diagnosis of tuberculous meningitis. Those who received both antitubercular and antipyogenic treatment and also those who were given antimalarials
during the first seven days of therapy were excluded from the study
group. Patients who expired during their hospital stay, those who absconded and those who left against
medical advice were also excluded from the study group. This was done purposely because response to therapy was the 'Gold standard' adopted in the study and lack of response could have resulted in error in classifying cases and controls. Fifty patients
who qualified for these. criteria were de- fined as "cases". Staging of the disease was done using British Medical. Council system(5). Out of fifty patients, 2 (4%) patients were in stage I, 17 (34%) in stage II, and 31 (62%) patients were in stage III. Forty eight patients of nontubercular CNS disorders. 31 patients of pyogenic meningitis (diagnosed by CSF findings and positive cultures and response to exclusive anti-pyogenic meningitis treatment); 10 patients of encephalitis; 2 patients of CT scan proved brain tumor (cerebellar astrocytoma and medulloblastoma)
and 5 patients of generalized tonic clonic seizures in whom no etiology
could be found despite extensive diagnostic workup were included as "controls".
A total of 98 serum and CSF samples from 50 cases with clinical diagnosis of tuberculous meningitis and 48 controls with CNS disorders of nontubercular etiology were subjected to immunodiagnostic tests under evaluation, namely, detection of
H37RV M. tuberculosis antigen in CSF by Dot ELISA, and detection of antibodies (IgG) against
H37RV strain of M. tuberculosis in CSF and serum by plate ELISA.
Laboratory Methods
Antigen Extraction
H37RV strain of Mycobacterium tuberculosis was cultured in Sauton's medium at
37oC for four weeks. The growth was subjected to heat
treatment for 15 minutes in boiling water bath to kill the cells
followed by centrifugation at 8000 rpm for 10 minutes. The cells were suspended in Tris 0.01 M(pH 7.0) and O.1M Phenyl Methyl Sulphonyl Fluoride (PMSF) and then were sonicated for a total of 45 minutes in cold at 20 Kilocycles (MSC Soni Prep.) Sonication was followed by centrifugation at 12000 rpm for 90 minutes and the supernatant with antigen was collected and stored
at -20°C. The protein concentration as estimated by Lowry's method was 1.8 mg/ml(5).
Antibody Detection by Plate ELISA
H37RV antigen in carbonate buffer (0.06 M, pH 9.6) (200
µl) was coated in microtitre plates (Tarsons) and incubated overnight at 4oC. Following morning, plates were washed with phosphate buffer saline (pH 7.3) containihg 0.05% Tween-20 (PBS-T) and non-specific sites were blocked with 30/0 Bovine Serum Albumin (BSA, Sigma Chemical Co, USA). After incubation for 90 minutes at 3TC, 200
µI of test sample diluted in PBS (serum 1:20 dilution, CSF 1:2 dilution) were added to each well. Each sample was tested in duplicate. After 90 minutes of incubation at 37°C plates were washed with (PBS-T) and 200
µI of 1:1000 diluted anti-human IgG-horse.
radish peroxidase conjugate (Sigma chemical Co., USA) . was added, followed by. incubation for 90 minutes at 3TC, and, after another wash, substrate (200 Ill) of Ortho Phenylene Oiamine (OPO, 40 mg/50 ml) in phosphate citrate buffer (pH 6.0) containing hydrogen peroxide was added and reaction was allowed to proceed at room temperature in dark for 15-20 minutes. Reaction was terminated by addition of 5 N sulphuric acid to each well and readings were taken by ELISA reader at 492 nm optical density.
Antigen Detection by Dot ELISA
This was performed using nitrocellulose paper (Schtcher & Schnell, Germany) on which CSF samples in 1:2 dilution were vacuum blotted in duplicate (Bioblot, Bionad). After washing with Tris buffer saline containing 0.5% Tween.20 (TBS-T) and blocking-of non-specific sites by 3% Bovine serum albumin for 90 min at 37oC, rabbit antibody to
H37RV at 1:800 dilution was added and again incubated at 37oC
for 90 minute. After washing with TBS-T, nitrocellulose paper was incubated again with 1:1000 diluted horse radish peroxidase
conjugated antirabbit IgG (Sigma Chemicals Co, USA) followed by washing and addition of substrate, 3-3 diaminobenzidine tetrahydrochloride (Sigma Chemicals
Co, USA) with 0.05% hydrogen peroxide. After 10-15 minutes of incubation
at room temperature, the strips were washed thrice with distilled water, and were allowed to dry. Results were recorded visually as positive or negative.
Statistical Analysis
Results of dot ELISA for detection of H37Rv antigen in CSF were recorded as dichotomous variable, namely, positive or negative. Results of detection of antibodies against H37Rv antigen in CSF
and serum by plate ELISA were recorded as optical densities ,(continuous
variable) compared
between various groups using ANOVA, and Mann Whitney test, as and where applicable.
Results of ELISA test were taken as positive when the value of mean
optical density obtained was more than 2 standard deviations above mean
for that test in controls. Sensitivity, specificity, positive/negative predictive values were computed as indices of applicability of diagnostic test under evaluation.
Results
Maximum children (38%) were in the age group of 1-3 years. The youngest and the oldest patients in which TBM was diagnosed were 8 months and 9 years old, respectively. Male to female ratio was 1.77:1. History of tubercular contact was
present in 38% (n=19) children. Fever was
the commonest presenting feature in 94%
(n=47), followed by convulsions in 64%
(n=37).
Acid fast bacilli could be demonstrated by direct smear examination and culture in
only 6% (n=3) CSF samples of patients of tuberculous meningitis. Twenty
five patients (50%) with tuberculous meningitis and 64.58% (n=31) patients of other
diseases were vaccinated with BCG. On tuberculin testing induration of more than
10 mm was noted in 52% (n=26) patients
with tuberculous meningitis while none of the children in control group were tuberculin positive.
Results of dot ELISA for detection of
H37RV
antigen in CSF were positive in 90% (n=45) cases of tuberculous meningitis, and 4.17% (n = 2) patients of CNS disorders
of nontubercular etiology [3.22% (n=1)
patients of pyogenic meningitis (due to N. meningitidis) and 50% (n=1) patient with brain tumor (cerebellar astrocytoma)] (Table I). Sensitivity of the detection of H37Rv antigen in CSF was 90% with specificity of 95.83%
(Table
II).
The mean
±SD (range) of optical densities obtained on plate ELISA for detection of antibodies against
H37RV antigen in CSF
and serum, and cutoff values for considering results of these two test to be positive,
are shown in
Table I
(Figs.
1 & 2). Optical
densities for both the tests were significantly higher in patients of clinically diagnosed tuberculous
meningitis as compared to patients of CNS disorders of non tubercular etiology (p <0.0001). Further, there was no statistically significant difference in optical densities among various sub-groups of controls (p > 0.05). Results of detection of antitubercular antibodies against
H37RV antigen in CSF were positive in 74% (n=37) cases of IBM, and 10.42% (n=5)
controls [in 6.45% (n=2) patients of
pyogenic meningitis, 10% (n=1) patient of encephalitis and 100% (both) patients of brain tumor) (Table I and Fig. 1). Sensitivity of the test was 74% and specificity was 89.58% (Table II).
Results of detection of antibodies against
H37RV antigen in serum was positive in 50% of cases and 8.33% of controls
[6.45% (n
=
2) patients of pyogenic meningitis and 20% (n
=
2) patients of encephalitis] thus making it
50% sensitive and
91.67% specific for diagnosis of TBM
(Tables
I & II, Fig. 2).
TABLE I
Results of Detection of Antige1ls (Ag) and Antibodies (Ab) in CSF and Serum of Cases and
Controls
Diagnosis (n)
|
Antigen in CSF
|
Antibody in CSF |
Antibody in Serum |
No. of
patients
with +ve results |
Mean±SD
(Range) |
No. of
patients
with +ve results |
Mean±SD
(Range) |
No. of
patients
with +ve results |
|
Cases |
|
|
|
|
|
|
TBM (N
=
50)
|
45 |
0.664±0.207
|
37 |
0.552±0.26
|
25 |
|
|
|
(0.28-1.20) |
|
(0.70-1.0) |
|
|
Controls (n
=
48)
|
2 |
0.225±0.153
|
5 |
.0.246±0.110
|
4 |
|
|
|
(0.06
- 0.6)
|
|
(0.11 - 0.53)
|
|
|
(i) |
Pyogenic meningitis |
1 |
0.213±0.145
|
2
|
0.253±0.098 |
2 |
|
(n
=
31)
|
|
(0.28-1.2) |
|
(0.70-1.0) |
|
|
(ii)
|
Encephalitis |
- |
0.203±0.149 |
1 |
0.267±0.157
|
2
|
|
(n
=
10)
|
|
(0.11-0.6) |
|
(0.12-0.52) |
|
|
(iii) |
Seizure disorder |
- |
0.200±0.067
|
-
|
0.156±0.046
|
|
|
(n=5)
|
|
(0.13-0.31) |
|
(0.1-0.21) |
|
|
(iv) |
Brain tumor* |
1 |
0.55,0.6 |
2 |
0.21,0.3 |
|
|
(n = 2) |
|
|
|
|
|
|
P value |
|
|
|
|
|
|
Cases vs. Controls |
|
< 0.0001 |
|
< 0.0001 |
|
|
Different control groups |
|
0.137 |
|
0.107 |
|
|
Cut off value** |
|
0.531 |
|
0.466 |
|
* Individual values; **Two standard deviations above mean values in control group.
TABLE II
Comparative Evaluation of Various Immunodiagnostic Tests for Diagnosis of TBM
Parameter
|
Antigen in CSF |
Antibody in CSF |
Antibody in Serum |
| Sensitivity (%) |
90.0 |
74.00 |
50.00 |
| Specificity (%) |
95.83 |
89.58 |
91.67 |
| Predictive value of a
+ve test (%) |
95.74 |
88.10 |
86.21 |
| Predictive value of a
-ve test (%) |
90.19 |
76.78 |
63.76 |
| % of false -ve test
(%) |
10.00 |
26.00 |
50.00 |
| % of false +ve test
(%) |
4.17 |
10.42 |
8.33 |
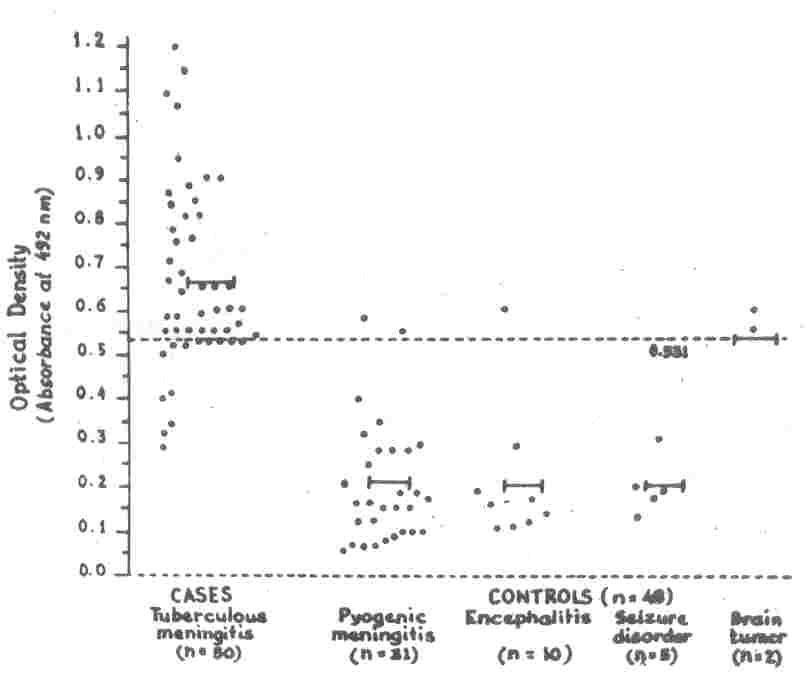 |
|
Fig 1. Detection of Antibodies in CSF in
Cases
and Controls.
Each dot in horizontal plane represents the absorbance value
recorded for individual patient. I-I Each line shows the mean optical density in respective groups.
- - - The broken line represents the cutoff
value (0.531) [250 above mean value in control group (all groups taken together) above which the test was considered to be positive].
|
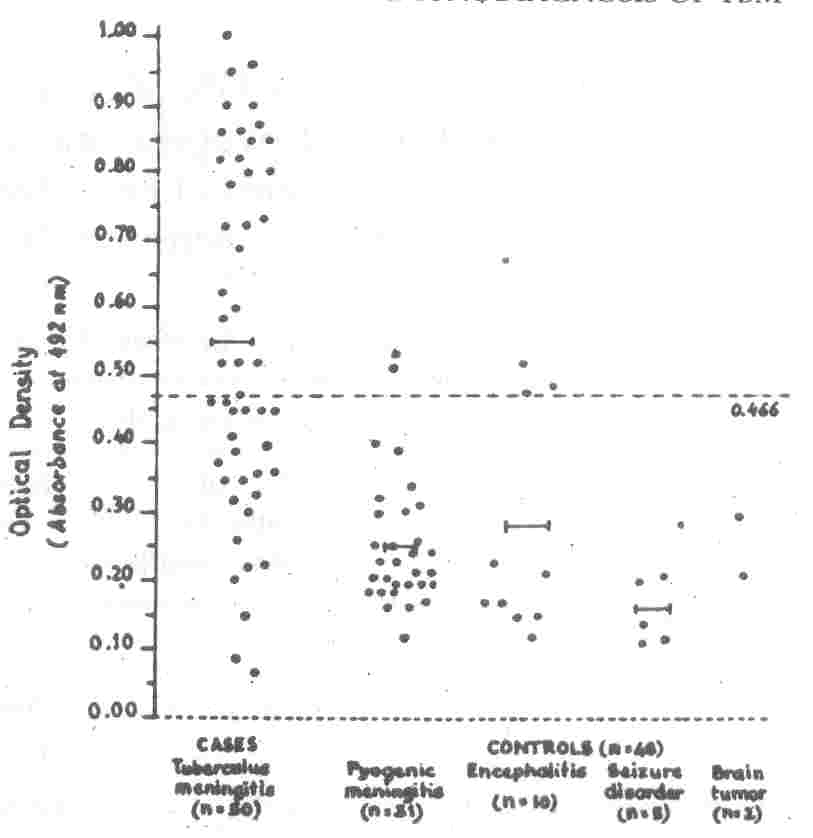 |
|
Fig. 2. Detection of Antibodies in Serum in Cases
and Controls. Each dot in horizontal plane represents the absorbance value recorded for individual patient.
I-I
Each line shows the
mean optical density in respective groups.
- - - The broken line" represents the cutoff
value (0.466) [2SD above mean value in control group (all groups taken together) above which the test was considered to be
positive]. |
Detection of antigen in CSF was found to have maximum sensitivity and
specificity (90% and 95.83%, respectively), positive as well negative
predictive values (95.74% and 90.19%, respectively), followed by detection of antibody in CSF
with positive predictive value of 88.10% and negative predictive value
of 76.78%. Detection of
antitubercular antibodies in serum was found to have the least positive as well negative predictive value (86.21% and
63.76, respectively)
(Table
II).
Discussion
We adopted the response to antitubercular therapy as the alternative gold standard for diagnosis of tuberculous
meningitis in the study. Bacteriological confirmation could be obtained in only 6% (n=3) clinically diagnosed cases of tuberculous meningitis who otherwise satisfied the criteria adopted in the study and responded
to exclusive antitubercular therapy. It might appear from the results that diagnosis of tuberculous meningitis can be made with certainty using the criteria adopted in the study but it should be realized that this highly selected studied sample does not truly represent the spectrum seen in hospital practice where a clinician often comes across situations where making a diagnosis is a real difficult task. The aim of the study was to evaluate the diagnostic potential of indirect, simple and inexpensive methods of diagnosis which may be applied in such cases posing diagnostic difficulties and should be interpreted considering this point. Alternative criteria, namely, CSF and CT scan findings, response to therapy, follow-up often have to substitute for 'Gold Standard' especially in tuberculous meningitis and have been used by other workers
in making diagnosis of tuberculous meningitis while evaluating a diagnostic test(7,8).
A large number of studies have been conducted with wide variation in the methodology employed by various workers in immunodiagnosis of tuberculous meningitis and a wide variety of antigens are available for immunoassay in tuberculosis(9-36). We used sonicated extract of
H37RV antigen for immunodiagnosis of tubercular etiology in our study.
H37RV strain of M. tuberculosis has been used by many workers for immunodiagnosis
of tuberculosis in different settings using varied methodology and yielded good results(14,20 ,22,31-35).
In the immunological spectrum of human tuberculosis, patients range from localized tuberculosis with well developed to those having poorly developed immunity and assay for detection of antigen and antibodies will depend on the position of the patient in the spectrum(37). The relative value of an ELISA system will be deter- mined by the preponderance of antigen, antibody or immune complexes in the CSF depending on the stage of the disease(36).
Results for detection of H37Rvantigen in CSF by dot ELISA were negative in 10% (n
=
5) cases who met the criteria for diagnosis of TBM adopted in the study. Levels of antigen below the detectable limits may
be possible explanation for these cases or it .could be that the manifestation of TBM were predominantly due to an Ag-Ab immune complex mediated reaction(21). Two positive results were obtained in 1 case of pyogenic meningitis in whom, N. meninigitidis was isolated on CSF culture and case of cerebellar astrocytoma. Detection of
H37RV antigen in CSF was found to be 90% sensitive and 95.83% specific for diagnosis of tuberculous meningitis.
The CSF antibody test was 74% sensitive and 89.61% specific for diagnosis of
TBM. Varying results have been demonstrated by other workers. Bal et al. detected antibodies to the same antigen as ours (H37Ry) in all the 9 patients of TBM with one false positive result in pyogenic meningitis(20). Sipsova et al. (Unpublished Abstract, 1990) reported sensitivity and specificity of detection of antibodies in CSF as 76.9% and 45% respectively. Samuel et al.
reported sensitivity of 66-74% and specificity of 100% when using radioimmunoassay technique with this antigen(31).
False positivity of detection of both antigen and antibodies in CSF of patients with pyogenic meningitis needs some explanation for its obvious importance. Cross reactions have been reported between polysaccharides of mycobacteria and other bacterial genera(38,39). A 65 Kba Ag of Mycobacteria known as 'heatshock protein' has been shown to be present in E. coli also, and appears to be present in a wide variety of other bacteria also(40). Among the 2 patients with brain tumor, both antigen and antibody were detected in CSF in the patients with cerebellar astrocytoma, whereas the other patient with medulloblastoma demonstrated presence of antigen only in CSF. We have no possible explanation for this. However, sharing of Ag between tumor cells and M. tuberculosis
antigen has been observed by other workers also(41,42).
The serum antibody test was found to be 50% sensitive and 91.67% specific for diagnosis of TBM. Krambovitis et al. reported a sensitivity of 84.5% and specificity of 84% for diagnosis of extra pulmonary tuberculosis(32). The false negativity in serum has also been explained by them on the basis of formation of immune complexes(32). Further, antibodies to
H37RV were detected in serum in 74% CSF samples from patients of tuberculous meningitis, whereas same antibody was detectable in serum in only
50% of these patients. This phenomenon of lower sensitivity of detection of serum anti- body as compared to detection of anti-bodies in body fluids has also been observed and explained by other workers according to whom specific antitubercular antibodies may be present in CSF in active disease but may not be found in serum(43).
No test can be expected to match the diagnostic accuracy of demonstration of tubercle bacilli in diagnosis of tubercular etiology of the disease but considering its low sensitivity and the difficulties encountered in clinical practice, alternate or indirect methods when employed with the complete clinical profile may be useful for therapeutic decision making process. The usefulness of immunodiagnosis
in different settings can be assessed by calculating predictive . value which vary with the prevalence of the disease under consideration in the geographical area where such studies are conducted. High prevalence rates lead to more true positive predictive accuracy. Similarly, lower prevalence rates lead to fewer false positive results and hence better negative predictive accuracy. In areas of high prevalence like India, a negative test
may be helpful to exclude mycobacterial disease from further consideration. How- ever, false negative tests results would be unacceptable in such situations and a very high sensitivity would be required.
Detection of antigen in CSF with the highest sensitivity (90%), specificity (95.83%), positive predictive. value (95.74%), as well as negative predictive value (90.19%) appears to be the most promising test for the diagnosis of tuberculous meningitis, followed by detection of antibodies in CSF, with slightly lower sensitivity (74%), specificity (89.58%) and positive and negative predictive values of 88.10% and 76.78%, respectively. Detection
of antibodies in serum with lowest sensitivity and lowest positive and negative predictive values does not appear to be much useful for this purpose, atleast in highly endemic areas like India. However, more studies involving larger number of patients need to be conducted to support and confirm these findings and before their routine use in initiating therapy is adopted or advocated.
|
1.
Manchanda SS, Lal H. Tuberculous meningitis in Children: A problem unsolved in India. Indian Pediatr 1966; 3: 167-176.
2.
Kapoor S. Evaluation of treatment of tuberculous meningitis since the use of corticosteroids as
adjuvants. Indian Pediatr 1970; 6: 166-171.
3.
Rattan A. Diagnosis II. Laboratory methods. In: Essentials of Tuberculosis in Children. Ed. Seth V. Jaypee Brothers, New Delhi, 1997; pp 222-33.
4.
Grange JM, Laszio A. Serodiagnostic tests for tuberculosis: A need for assessment of their operational predictive accuracy and acceptability. Bull WHO 1990; 68: 571-576.
5. Medical Research Council. Streptomycin treatment of tuberculous meningitis:
Report of the Committee on Streptomycin in TB Trial. Lancet 1948; 1: 582-596.
6.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with
Folin-phenol reagent.
J
Bioi Chern 1951; 193: 265-275.
7.
Ahuja GK, Mohan KK, Behari M. Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis 1994; 75: 149-152.
8.
Kumar R, Kohli N, Thavnani H, Kumar A, Sharma B. Value of CT scan in the diagnosis of meningitis. Indian Pediatr 1996; 33: 465-468.
9.
Hernadez R, Munoz 0, Guiz-cafre H. Sensitive enzyme immunoassy fro early diagnosis of TBM.
J
Clin Microbiol 1984; 20: 533-535.
10.
Coovadia YM, Dawood, A, Eilis ME, Coovadia HM, Daniel TM, Evaluation of adenosine deaminase activity and anti-body to M. tuberculosis antigen 5 in cerebrospinal fluid for the early diagnosis of tuberculous meningitis. Arch Dis Child 1986; 61: 428-435.
11.
Watt G, Zaraspe G, Bautista S, Laughlin LW. Rapid diagnosis of tuberculous meningitis by using an enzyme linked immunosorbent assay to detect myco-bacterial antigen and antibody in cere-brospinal fluid.
J
Infect Dis 1988; 158: 681- 686.
12.
Dole M, Maniar P, Lahiri K, Shah MD. Enzyme Linked Immuno Sorbent Assay for the detection of M. tuberculosis specific IgG antibody in the cerebrospinal fluid in
cases of tuberculous meningitis.
J
Trop Pediatr 1989; 35: 218-220.
13.
Chandramuki A, Bothamley GH, Brennan PJ, Ivanyi J. Level of antibody to defined antigens of M. tuberculosis in tuberculous meningitis.
J
Clin Microbiol1989; 27: 821- 825.
14.
Kadival G, Samuel AM, Virdi BS, Kale RN, Ganatra RD. Radioimmunoassay of
tuberculous antigen. Indian
J
Med Res
1982; 75: 765-770.
15.
Sarala R, Raja A. Antibody to PPD, H37Rv
and antigen-5 in tuberculous meningitis.
J
Trop Pediatr 1991; 37: 266-267.
16.
Park SC, Lee BI, Cho SN, Kin WJ, Lee BC, Kim SM, et al. Diagnosis of tuberculous meningitis by detection of immunoglobulin G antibodies to purified derivative and lipoarabinomannan antigen in cerebrospinal fluid. Tuber Lung Dis 1993; 74: 317-322.
17.
Miorner H, Sjobring V, Nayak P, Chandramuki A. Diagnosis of tubercu- 'lous meningitis. A comparative analysis of 3 immunoassy, an immune complex as- say and the polymerase chain reaction. Tuber Lung Dis 1995; 76: 381-386.
18.
Maniar P, Joshi L. ELISA - its evaluation in diagnosis of tuberculous meningitis.
Indian
J
Pediatr 1990; 57: 667~672.
19.
Sada E, Ruiz-Palacios GM, Opez Vidaly L, Ponce De Le ONS. Detection of mycobacterial antigens in cerebrospinal fluid of patients with tuberculous meningitis by enzyme-linked immunosorbent assay. Lancet 1983; 2: 651-652.
20.
Bal V, Kamat RS, Kamat J, Kandoth P. Enzyme linked immunosorbent assay for
mycobacterial antigens. Indian
J
Med Res 1983; 78: 477-483.
21.
Chandramuki A, Allen PRJ, Keen M, Ivanyi
J.
Detection of Mycobacterial antigen and antibodies in the CSF of patients with tuberculous meningitis.
J
Med Microbiol1985; 20: 239-247.
22.
Wagle N, Vaidya A, Joshi S, Merchant SM. Detection of tubercle antigen in cerebrospinal fluids by ELISA for diagnosis of tuberculous meningitis. Indian
J
Pediatr 1990; 57: 679-683.
23.
Radhakrishnan VV, Mathai A. Detection of M. tuberculosis antigen-5 in CSF
by inhibition ELISA and its diagnostic potential in TBM.
J
Infect Dis 1991; 163: 650- 652.
24.
Radhakrishnan VV, Mathai A. Detection of Mycobacterial antigen in cerebrospinal fluid in patients with chronic meningitis by inhibition enzyme linked immuno sorbent assay. Indian
J
Med Res 1990; 91: 355-359.
25.
Ivanyi J, Bothamley GH, Jackett PS. Immunodiagnostic assay for tuberculosis and leprosy: Br Med Bull 1988; 44: 635- 649.
26. Singh M, Andersen AB, McCarthy JE, Rohde M, Schutte H, Sanders E, et al.
The
M. Tuberculosis 38 KDa antigen overproduction in Escherichia coli, purification and characterization. Gene 1992; 117: 53- 60.
27.
Young DB, Kaufman SHE, Hermans PWM, Thole JER. Mycobacterial protein
antigens: A complication. Mol Microbiol 1992; 6: 133-145.
28.
Ramkisson A, Coovadia VM, Coovadia HM. A competition ELISA for the detection of mycobacterial
antigen in tuberculous exudates. Tubercle 1988; 69: 209-212.
29.
Lee BY, Heftier AS, PJ. Characterization of the major membrane proteins of virulent M. tuberculosis. Infect. Immunity 1992; 60: 2066-2074.
30.
Khanolkar-Youngj S, Kolk AH, Anderson AB, Bennedsen J, Brennan PJ, et al. Results of the Third Immunology of Leprosy/Immunology of Tuberculosis Antimyco-bacterial Monoclonal Antibody Workshop. Infect Immunity 1992; 60: 3925- 3927.
31.
Samuel AM, Kadival GV, Invany J, Pandya SK, Ganatra RD. A sensitive and specific method for diagnosis of tuberculous meningitis. Indian
J
Med Res 1983;
77: 752-757.
32.
Krambovitis E, Harris M, Hughes DTD. Improved serodiagnosis of tuberculosis
using mycobacterial antigens. Indian
J
Med Res 1983; 78: 477-483.
33.
Mehta PK, Khuller GK. Serodiagnostic potentialities of ELISA using monophosphoinosides of M. tuberculosis
H37RV Med Microbiol Immunol Berl1986; 77: 285-292.
34.
Stavri D, Niculescu D, Stavri H, Borea F, Stancia G, Bosacopol A. Humoral immune response in human pulmonary tuberculosis:
Antibodies against Mycobacterium tuberculosis polysaccharide protein and glycolipid antigens. Roum Arch Microbiol Immunol1993; 52: 109-119.
35.
Desai T, Gogate A, Deodhar L, Toddywalla S, Kelkar M. Enzyme linked
immuno sorbent assay for antigen detection in tuberculous meningitis Indian
J Pathol Microbiol 1993; 36: 348-355.
36.
Mahadevan S. Diagnosis III. ELISA in tuberculous meningitis. In: Essentials of Tuberculosis in Children. Ed. Seth V. New Delhi, Jaypee Brothers, 1997; pp 234-239.
37.
Kalish SB, Radin RC. Use of enzyme linked immunoassay techniques in differential diagnosis of active tuberculosis in
humans.
J
Infect Dis 1983;147: 523-530.
38.
Minden P, McClatchy JK, Farr RS. Shared
antigens between heterologous bacterial species. Infect Immun 1972; 6; 574-582.
39.
Minden P, McClatchy JK, Cooper R, Bardana EJ Jr, Farr RS. Shared antigens between Mycobacterium bovis (BCG) and other bacterial species. Science 1972; 176: 57-58.
40.
Rattan A, Sriniwas. Possible reasons for false positive ELISA results in serodiagnosis of tuberculosis. Indian Pediatr 1990; 27: 881-882.
41.
Minden P, Sharpton TR, McClatchy JK, Shared antigens between human malignant melanoma cells and Mycobacterium bovis (BCG). J Immunol 1976; 116: 1407- 1414.
42.
Dhand R, Ganguly NK, Vaishnavi C. Gilhotra R, Malik SK. False-positive reactions with enzyme-linked immunosorbent assay of Mycobacterium tuberculosis antigens in pleural fluid.
J
Med Microbiol. 1988; 26: 241-243.
43.
Kinnman I, Link H, Fryden A. Characterisation of antibody activity in oligoclonal immunoglobulin G, syhthesized within the central nervous system in a patient with tuberculous meningitis.
J Clin Microbiol1981; 13: 30-35.
|
