|
|
|
Indian Pediatr 2021;58: 994-996 |
 |
Anakinra in Refractory Multisystem
Inflammatory Syndrome in Children (MIS-C)
|
|
Chandrika S Bhat,1* Rakshay Shetty,2 Deepak Ramesh,2
Afreen Banu,2 Athimalaipet V Ramanan3
From 1Pediatric Rheumatology Services, and 2Pediatric
Intensive Care Services, Rainbow Children’s Hospital, Bangalore,
Karnataka, India; 3Bristol Royal Hospital for Children and Translational
Health Sciences, University of Bristol, United Kingdom.
Email:
[email protected]
|
|
A small proportion of children can develop a hyper-inflammatory
condition 2 to 4 weeks following an infection or exposure to SARS-CoV2
virus termed interchangeably as multisystem inflammatory syndrome in
children (MISC) or Pediatric Inflammatory Multisystem Syndrome
temporally associated with SARS-CoV-2 virus (PIMS-TS) [1]. The patho-genesis
of this novel condition remains elusive and treatment protocols are
predominantly empirical. Intravenous immuno-globulin (IVIG) alone or
with corticosteroids are the suggested first line agents [2,3]. In those
with refractory disease (defined by the presence of persistent fever
and/or significant end-organ involvement despite initial
immunomodulation), second line treatment options include IL-1, IL-6, and
tumor necrosis factor (TNF) blockers [2,3]. The PIMS-TS arm of the
RECOVERY trial is currently evaluating tocilizumab and anakinra for
refractory disease [5]. The experience with use of anakinra in India is
sparse due to non-availability of the drug. We report our experience
with the use of anakinra in two children with refractory MISC.
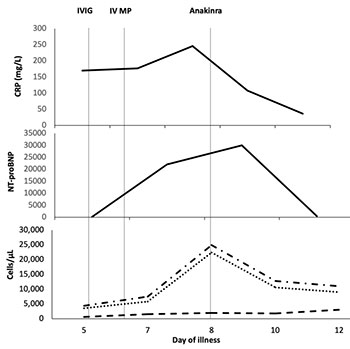
Case 1: A 11-year-old boy was referred for fever,
abdominal pain and diarrhea of 5 days duration. At presentation he was
hypotensive (BP 70/40 mm Hg) with bilateral non purulent conjunctival
suffusion and an erythematous maculopapular rash over his trunk.
Investigations revealed lymphopenia (total white blood cell count (WBC)
4,350/ìL, lymphocytes 4%), elevated inflammatory markers (CRP 170 mg/L,
ESR 72 mm/hour, LDH 359 U/L, ferritin 1,200ng/mL, d-Dimer 12,500ng/mL),
hyponatremia (130mEq/L) and increased NT-proBNP levels (>20,000pg/mL).
Serology was positive for IgG SARs-CoV-2 antibodies (Chemiluminescence,
titer 74.9 AU/mL). An echocardiogram showed decreased left ventricular
function (LVEF, 40%). A diagnosis of MIS-C was considered, and he was
given IVIG (2 g/kg) with intravenous methylprednisolone (IVMP) (2
mg/kg). Noradrenaline infusion (0.15 µg/kg/min) for hypotension and
empirical antibiotics were commenced simultaneously. The dose of
methylprednisolone was increased (10 mg/kg, once daily for three days),
and adrenaline infusion (0.15 µg/kg/min) was started for persistent
hypotension. He continued to be febrile and hypotensive with elevated
inflammatory markers (Fig. 1a). Considering refractory disease,
anakinra (5 mg/kg/day in two divided doses, subcutaneously) was
initiated. There was a dramatic improvement in his clinical status with
abrupt cessation of fever and normalization of blood pressure within 12
hours. LVEF increased subsequently (60%). Anakinra was discontinued
after 48 hours, and he was discharged on a tapering dose of steroids and
aspirin. On follow up at two- and six weeks, LVEF continued to be
normal.
 |
 |
|
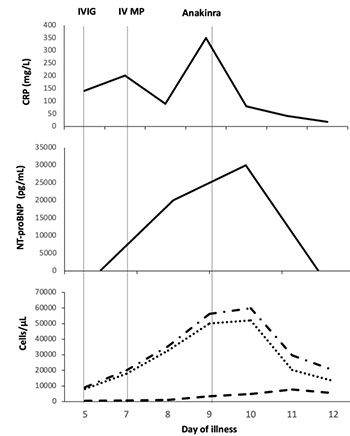
IVIG- intravenous immunoglobulin (2 g/kg),
IVMP- intravenous methylprednisolone (10mg/kg once daily)
Fig. 1 Trend of inflammatory markers and therapeutic
interventions in (a) Case 1 and (b) Case 2.
|
Case 2: A 9-year-old boy presented with fever and
diarrhea of five days. On examination, he had bilateral conjunctival
suffusion, red lips, strawberry tongue, erythematous maculo-papular
rash, and pedal edema. Investigations revealed lymphopenia (WBC count
9,230/µL, lymphocyte 6%), elevated acute phase reactants (CRP 140 mg/L,
ESR 60 mm/hr, ferritin 596 ng/mL, d-Dimer 9120 ng/mL), and hyponatremia
(127 mEq/L). He tested positive for IgG SARs-CoV-2 anti-bodies (Biomerieux,
Index 10.95). An echocardiogram showed dilatation of the right coronary
artery (RCA, z-score 2.19). His presentation was consistent with
Kawasaki disease like phenotype of MISC and he was given IVIG (2 g/kg)
with IVMP (2 mg/kg/day). On day 3 of admission, fever recurred, and he
developed hypotension necessitating inotropic support (noradrenaline,
0.15 µg/kg/min). Inflammatory markers and NT-proBNP levels had further
increased (Fig. 1b). Repeat echocardiogram showed a decrease in
LVEF (35%) and progression of RCA involvement (z-score 3.5). The dose of
methylprednisolone was increased to 10 mg/kg once daily for 3 days. On
day 5 of admission, his inotropic requirement (adrenaline, 0.5
µg/kg/min) and inflammatory markers progressively increased. Anakinra (6
mg/kg/day in two divided doses, subcutaneously) was commenced for
refractory disease. Within 48 hours, he was off inotropic support with
defervescence of fever, down trending inflammatory markers and normal
LVEF (60%). He was discharged on a slow taper of oral steroids and
aspirin. On follow up at two- and six weeks, coronary vessels and LVEF
were within normal limits.
Anakinra is a recombinant IL-1R antagonist that
blocks the binding of both IL-1 a
and IL-1b to
IL-1R, thereby inhibiting the proinflammatory effects of IL-1. According
to the American College of Rheumatology clinical guidance, anakinra
(4-10 mg/kg/day) is the preferred monoclonal antibody in refractory
MIS-C [4]. However, the United Kingdom national consensus guidance
recommends tocilizumab, anakinra or infliximab depending on clinician
preference [3]. In comparison to tocilizumab or infliximab, the short
half-life of anakinra makes it more favorable for use in the Indian
context where secondary infections are a cause of concern. In fact,
anakinra was found to be beneficial in patients with severe
sepsis, especially in the subset with macrophage activation syndrome
[6]. The cost of treatment with anakinra compares favorably with
tocilizumab. Our experience re-emphasizes that anakinra can be an
effective therapeutic agent in children with MISC who do not respond to
IVIG and corticosteroids.
REFERENCES
1. Bhat CS, Gupta L, Balasubramanian S, Singh S,
Ramanan AV. Hyperinflammatory syndrome in children associated with
COVID-19: Need for awareness. Indian Pediatr. 2020;57: 929-35.
2. Fouriki A, Fougère Y, De Camaret C, et al.
Case series of children with multisystem inflammatory syndrome following
SARS-CoV-2 infection in Switzerland. Front Pediatr. 2021;8:594127.
3. Harwood R, Allin B, Jones CE, et al. PIMS-TS
National Consensus Management Study Group. A National Consensus
Management Pathway For Paediatric Inflammatory Multisystem Syndrome
Temporally Associated With COVID-19 (PIMS-TS): Results of a National
Delphi Process. Lancet Child Adolesc Health. 2021;5:133-41.
4. Henderson LA, Canna SW, Friedman KG, et al.
American College of Rheumatology Clinical Guidance for Multisystem
Inflammatory Syndrome in Children Associated With SARS-CoV-2 and
Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol.
2021;73:e13-e29.
5. Nuffield Department of Population Health.
Information for site staff 2020. Accessed June 21, 2021. Available from:
https://www.recoverytrial .net/for-site-staff
6. Shakoory B, Carcillo JA, Chatham WW, et al.
Interleukin-1 receptor blockade is associated with reduced mortality in
sepsis patients with features of the macrophage activation syndrome:
Re-analysis of a prior phase III trial. Crit Care Med. 2016;44:275-28.
|
|
|
 |
|

