Summary
In this open-label, randomized controlled
trial in seven tertiary neonatal intensive care units in India,
Sri Lanka, and Bangladesh, infants born at or after 36 weeks of
gestation with moderate or severe neonatal encephalo-pathy and a
need for continued resuscitation at 5 min of age or an Apgar
score of less than 6 at 5 min of age (for babies born in a
hospital), or both, or an absence of crying by 5 min of age (for
babies born at home), were recruited. In a web-based
randomization system, infants were allocated into a group
receiving whole body hypothermia (33·5°C) for 72 h using a
servo-controlled cooling device, or to usual care (control
group), within 6 h of birth. All recruiting sites had facilities
for invasive ventilation, cardiovascular support, and access to
3 Tesla MRI scanners and spectroscopy. The primary outcome was a
combined endpoint of death or moderate or severe disability at
18-22 months, assessed by the Bayley Scales of Infant and
Toddler Development (third edition) and a detailed neurological
examination. Analysis was by intention to treat. After
exclusions, 202 eligible infants were assigned to the
hypothermia group and 206 to the control group. Primary outcome
data were available for 195 (97%) of the 202 infants in the
hypothermia group and 199 (97%) of the 206 control group
infants. 98 (50%) infants in the hypothermia group and 94 (47%)
infants in the control group died or had a moderate or severe
disability (risk ratio 1·06; 95% CI 0·87–1·30; P=0·55).
84 infants (42%) in the hypothermia group and 63 (31%; P=0·022)
infants in the control group died, of whom 72 (36%) and 49 (24%;
P=0·0087) died during neonatal hospitalisation. Five
serious adverse events were reported: three in the hypothermia
group (one hospital readmission relating to pneumonia, one
septic arthritis, and one suspected venous thrombosis), and two
in the control group (one related to desaturations during MRI
and other because of endotracheal tube displacement during
transport for MRI). Therapeutic hypothermia did not reduce the
combined outcome of death or disability at 18 months after
neonatal encephalopathy in low-income and middle-income
countries, but significantly increased death alone. The authors
conclude that therapeutic hypothermia should not be offered as
treatment for neonatal encephalopathy in low-income and
middle-income countries, even when tertiary neonatal intensive
care facilities are available.
Commentaries
Evidence-based Medicine Viewpoint
Introduction: Therapeutic hypothermia
(TH) is widely practiced in new-born infants with hypoxemic
ischemic encephalopathy (HIE). It has been included as a
standard of care in many guidelines published in developed as
well as developing countries. Its use has become so widespread
that the International Liaison Committee on Resuscitation
(ILCOR) statement in 2020 cautioned that TH should only be used
in neonatal care units with facilities for multidisciplinary
care, respiratory support, oxygenation monitoring, etc. [1]. TH
appears to be supported by robust evidence. A network
meta-analysis of randomized controlled trials (RCT) examining
multiple interventions for HIE [2], identified whole-body
cooling as the top-ranking intervention that reduced mortality
at 18 months of age, closely followed by selective head cooling.
Both interventions were also associated with better neuro-developmental
outcomes at that age. Even cerebral palsy in later life was
found to be decreased with TH [3].
Despite the overall benefit reported with TH,
it is not always successful, particularly in severe HIE. Perhaps
this is why there is intense search for alternate interventions
for neuroprotection and/or improvement of neuro-develop-mental
outcomes following neonatal encephalopathy. Several
interventions have been explored with and without TH, including
erythropoietin [4,5], melatonin [6,7], and xenon [8]. There are
also several pre-clinical studies as well as registered human
RCTs exploring stem cell therapy [9,10]. These diverse data
suggest that there is room for further evidence despite the
reported benefits of TH. Recently, a multi-centric RCT in three
developing countries, evaluated TH in moderate-to-severe HIE
[11]. Table I summarizes the trial details.
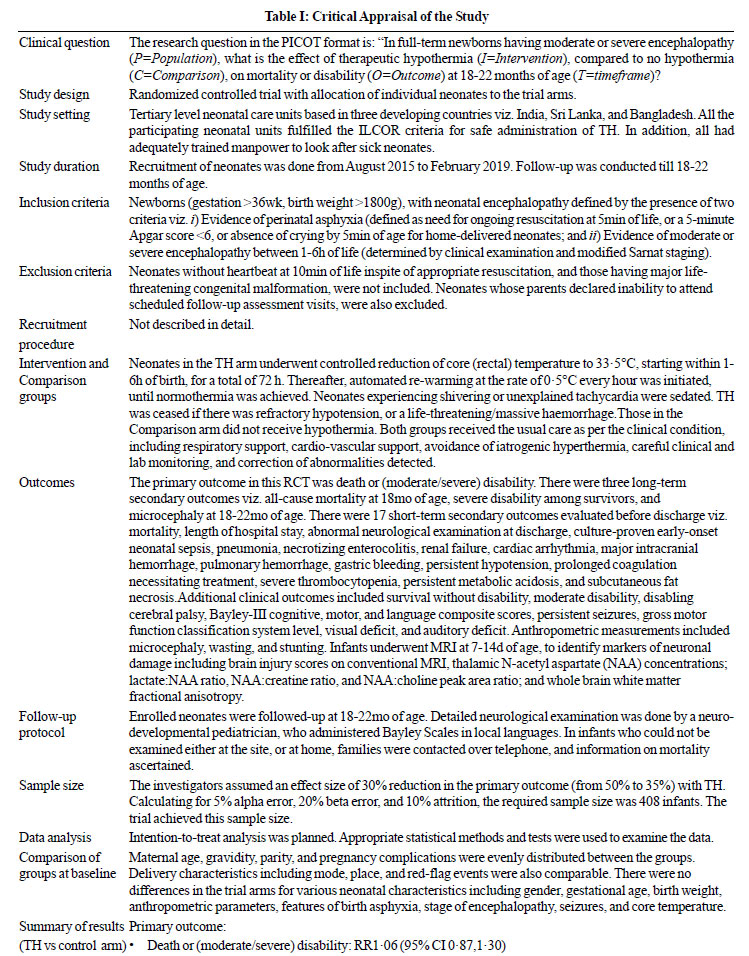 |
 |
Critical appraisal: Overall, the trial
[11] had low risk of bias. The random sequence was generated
using an online program that controlled for the stage of
encephalopathy as well as study site. Random permuted blocks of
variable sizes were used, although the range of block sizes was
not specified. Allocation was concealed from the on-site
investigators, who had to enter participant details after
informed consent was obtained, to identify the arm to which the
neonate was allocated. Adherence to the assigned arm, was
cross-verified by a team based in London. Although the treating
physicians/teams were not blinded, the assessors recording the
primary outcome, long-term outcomes, and the MRI data were
blinded to the allocation of each neonate. A wide range of
clinically important outcomes were recorded, without omitting
any from reporting. There was very low attrition in this trial,
as 97% of the enrolled infants could be followed-up. This RCT
[11] was not only registered, but its protocol was also
published [12], and there are no significant deviations from
either. Even after randomization, there were hardly any protocol
deviations.
The trial [11] included several refinements
in addition to meticulous planning, execution and analysis. This
enabled the investigators to overcome many biases that crept
into previous similar trials. For example, neonates who
underwent passive cooling prior to randomization were not
included. Variability in assessments that could creep into
clinical examinations, neuro-developmental evaluation, etc. were
diminished, because these were performed by well-experienced
physicians, and stringent definitions were used for every
subjective evaluation. Even the MRI scanning procedure,
protocols, and acquisition time, were standardized across the
sites. Raw data from MR scanning were centrally evaluated for
quality before processing. Two experienced neonatal neurologists
used a prior-validated scoring system to read the images, while
blinded to all clinical information.
In addition to the clinical outcomes, the
investigators included a large number of MRI-related parameters.
The choice of these is supported by a systematic review [13]
which confirmed that ratios of NAA/creatine and NAA/choline in
the basal ganglia/thalamus, as well as myo-inositol/choline in
the cerebral cortex on Proton magnetic resonance spectroscopy,
correlated well with adverse effects in neonates undergoing TH.
Similarly, MRI findings of injury to the internal capsule
posterior limb (on diffusion weighted imaging), and increased
lactate/N-acetylaspartate peak on MR spectroscopy, had high
predictive value for adverse neurodevelopmental outcomes [14].
All these were analyzed in this trial [11].
The extremely low attrition in this RCT [11]
was achieved by research nurses maintaining contact with the
families of enrolled infants between discharge and follow-up.
Special search teams were constituted to track families who
failed to follow-up as scheduled. These teams were able to make
home visits not only to local families, but even to those who
had migrated.
Very few limitations could be identified in
this trial [11], none of them serious. For example, although the
analysis was described as intention-to-treat, the calculations
were based on the number whose primary outcome was available,
rather than the number randomized. As in many multi-centric
trials, only aggregated data across study sites was presented,
making it difficult for readers to judge whether data are driven
by experiences in a limited number of sites with larger
proportion of enrolments. This is important because in this
trial [11], two sites accounted for 55% of the enrolled
neonates, whereas 3 sites, each enrolled less than 10% of the
sample size. One site enrolled only 12 neonates.
Since many of the enrolled neonates had
clinical seizures, it can be argued that EEG data would be
important. A systematic review showed that abnormal amplitude
integrated electroencephalogram (aEEG) at 72 hours had high
reliability to predict death or moderate/severe disability [15].
Another systematic review of 37 publications also confirmed that
aEEG at 24 and 72h, had high predictive value for adverse
neurodevelopmental outcomes [14]. However, this RCT [11] did not
perform EEG.
The robust methodology and multiple
refinements in this trial [11] generate high level of confidence
in the results. There was no difference between the RCT arms for
the primary outcome. Among the long-term outcomes, all-cause
mortality at 18 months was increased with no benefit in the
other two outcomes. Among the 17 short-term outcomes, 7 were
worse in TH group, with no benefit in the other 10. Among 13
additional clinical outcomes, only one viz. disabling cerebral
palsy showed a statistically significant reduction with TH,
whereas there was no difference in the other 12. The multiple
MRI findings were all comparable between the groups. In addition
to the outcomes presented above, the supplementary files [11],
have a plethora of additional data including hematological
parameters, biochemical values, and clinical support
requirements, recorded at 24h, 48h, 72h, and 96h. A wealth of
MRI data (too extensive to present here) is also included.
Overall, none of these showed any benefit of TH.
The authors also undertook multiple subgroup
analyses of one secondary outcome "mortality at discharge."
Three comparisons stood out. First, the increased mortality at
discharge was driven by outborn neonates. Surprisingly, there
was increased mortality in the TH group, among infants without
sepsis, and those having no perinatal sentinel events. It is
unclear why the primary outcome was not similarly analyzed.
Given that this methodologically robust RCT
[11] showed contrary results to several other studies, (thereby
challenging the hitherto accepted practice of TH in HIE),
several questions emerge.
First, how do the results of this trial [11]
compare with other data? A Cochrane review published in 2013 (11
RCT, 1505 participants) demonstrated statistically and
clinically important reduction in mortality or major neuro-developmental
disability at 18 months of age [16]. However, this review is
outdated and merits no further consideration. A very recent
systematic review with literature search updated to April 2020
[17], identified 28 RCTs among nearly 3600 neonates with
moderate to severe HIE. Meta-analysis showed that the pooled
relative risk of mortality was (statistically and clinically)
significantly reduced with TH. However, in addition to some
methodological flaws, the authors did not specify the time-frame
at which mortality was determined [17]. This makes it difficult
to interpret the data from the review [17]. On the plus side,
the authors did not identify significant publication bias (i.e.
lower probability of publication of trials showing no beneficial
effects of TH).
Since the publication of the systematic
review [17], additional trials have emerged. A recent RCT
conducted in Chennai [18] examining the same outcomes as this
trial [11] in over 160 neonates, reported a statistically
significant difference in mortality or abnormal neurological
outcome, at 18mo, although there was no significant difference
within 28d. Another RCT [19] in a single Indian institution
among 50 neonates with moderate or severe HIE, examining MRI
changes in the posterior limb of the internal capsule, reported
a statistically significant beneficial effect with TH, although
this could be analyzed in less than half the recruited infants.
Conventional MRI findings also suggested that TH was beneficial.
Yet another RCT among 120 neonates with HIE at JIPMER Puducherry,
reported lower mortality with TH [20], and also less frequency
and severity of acute renal injury. Markers of myocardial injury
(cardiac enzyme levels at 72h) and ECG as well as
echocardiography findings were more favorable in those receiving
TH [21]. An RCT in 40 Chinese infants [22], also reported lower
incidence of severe disability, better psychomotor development
scores, and higher neuro-development scores at 15 months of age
in those receiving TH. These infants also had better neonatal
neuro-behavioural score at 28 days of age. However, there was no
difference in mortality and no difference in the levels of
neuronal biomarkers after 72 hours of treatment. Overall, none
of these RCTs had the methodological rigour associated with this
trial [11].
Despite the overall benefit reported in
systematic reviews of TH [16,17], not all trials showed the same
effect. Even trials showing benefit differed in its magnitude. A
group of authors tried to analyze the reason for statistically
significant differences in the efficacy of TH in two fairly
large trials [23]. Despite similar inclusion criteria, there
were differences in the sickness level of included neonates,
severity of HIE, use of anti-convulsant medication, sedation,
and many in one of the trials had received cooling before
randomization itself.
To be fair, this is not the first robust
piece of evidence that failed to find a beneficial effect of TH.
A systematic review focusing on studies conducted only in
low-and middle-income countries, identified 7 trials [24]. These
trials included 567 infants, of whom 15% had only mild
encephalopathy. Various formal and non-formal cooling systems
were used. However, there was no statistically significant
decrease in neonatal mortality with TH. The authors attributed
this to heterogeneity, poor methodo-logical quality,
inappropriate cooling devices, or inadequate intensive care
facilities. However, they also considered population-based
differences (compared to high-income countries) such as
perinatal infection, obstructed labor, intrauterine growth
retardation, etc.
There are other indirect pieces of evidence
suggesting limitations to the effects of TH. A community-based
study in the UK followed up 145 survivor children, 6-7 years
after being randomized to TH or otherwise, to determine their
health-related quality of life (HRQL) [25]. However, no
statistically significant differences were observed. A similar
analysis on healthcare resource utilization and costs among 130
survivors aged 6-7 years (from the same cohort), showed lower
resource utilization in the TH arm, though the differences were
not statistically significant [26]. Another indirect evidence is
that hypothermia for longer than 72 hours, cooling to
temperature lower than 33.5°C, or both together, did not add
further benefit in terms of mortality or severe disability at 18
months of age [27,28].
The second important question is, what could
be the explanation for the results of this trial being
remarkably different? One possible explanation is that previous
trials often included neonates with mild HIE also, whereas this
trial [11] included only those with moderate or severe HIE. In
this context, a systematic review [29] identified 13 studies
wherein almost one in six included neonates had mild HIE. On
meta-analysis, about 22% of the infants who underwent TH had
only mild HIE. Another systematic review also identified 117
babies with mild HIE who had been inadvertently included in 5 TH
trials [30].
Another potential explanation is that, the
mechanism (and consequences) of perinatal asphyxia in
low-resource settings may be different from developed country
settings. In this context, the trial authors [11] themselves
suggested that the included babies underwent subacute, or
partial prolonged hypoxia (based on MRI findings). Further, the
occurrence of seizures in many infants in this trial [11]
suggested intra-partum hypoxia before birth, which could reduce
the neuro-protective effects of TH. There is also data that,
among neonates with birth asphyxia, the presence of hyperoxemia
at admission increases the risk of HIE [17]. This is referred to
as the oxygen paradox, wherein excess oxygen supplementation
following hypoxia worsens the outcome. In this trial [11], over
70% enrolled neonates were born at other institutions, wherein
less-skilled physicians may have used excess oxygen to manage
the hypoxia. One wonders whether this could be a contributing
factor.
Conclusion: This very well-designed and
well-executed landmark RCT confirmed that therapeutic
hypothermia (for 72h) in full-term neonates having moderate or
severe encephalopathy did not reduce the composite outcome of
mortality or disability at the age of 18-22 mo. On the contrary,
short-term, as well as long-term mortality were increased.
Several other clinically important outcomes were also worse in
those receiving TH, making it a harmful intervention. An urgent
review of the clinical practice of offering TH is warranted at
the institutional, as well as national levels.
Funding: None; Competing interests:
None stated.
Joseph L Mathew
Department of Pediatrics, PGIMER, Chandigarh.
dr.joseph.l.mathew@gmail.com
References
1. Wyckoff MH, Wyllie J, Aziz K, et al.
Neonatal life support: 2020 International Consensus on
Cardio-pulmonary Resuscitation and Emergency Cardiovascular
Care Science With Treatment Recommendations. Circulation.
2020; 142 (Suppl 1):S185-221.
2. Lee CYZ, Chakranon P, Lee SWH.
Comparative efficacy and safety of neuroprotective therapies
for neonates with hypoxic ischemic encephalopathy: a network
meta-analysis. Front Pharmacol. 2019; 10:1221.
3. Shepherd E, Salam RA, Middleton P, et
al. Neonatal interventions for preventing cerebral palsy: an
overview of Cochrane Systematic Reviews. Cochrane Database
Syst Rev. 2018;6:CD012409.
4. Ivain P, Montaldo P, Khan A, et al.
Erythropoietin monotherapy for neuroprotection after
neonatal encephalo-pathy in low-to-middle income countries:
a systematic review and meta-analysis. J Perinatol. 2021 Jun
26. [Epub ahead of print]
5. Razak A, Hussain A. Erythropoietin in
perinatal hypoxic-ischemic encephalopathy: a systematic
review and meta-analysis. J Perinat Med. 2019;47:478-489.
6. Jerez-Calero A, Salvatierra-Cuenca MT,
Benitez-Feliponi Á, et al. Hypothermia Plus Melatonin in
Asphyctic Newborns: A Randomized-Controlled Pilot Study.
Pediatr Crit Care Med. 2020;21:647-55.
7. Ahmed J, Pullattayil S AK, Robertson
NJ, More K. Melatonin for neuroprotection in neonatal
encephalopathy: A systematic review & meta-analysis of
clinical trials. Eur J Paediatr Neurol. 2021;31:38-45.
8. Rüegger CM, Davis PG, Cheong JL. Xenon
as an adjuvant to therapeutic hypothermia in near-term and
term newborns with hypoxic-ischaemic encephalopathy.
Cochrane Data-base Syst Rev. 2018;8:CD012753.
9. Serrenho I, Rosado M, Dinis A, et al.
Stem cell therapy for neonatal hypoxic-ischemic
encephalopathy: a systematic review of preclinical studies.
Int J Mol Sci. 2021; 22:3142.
10. Bruschettini M, Romantsik O, Moreira
A, et al. Stem cell-based interventions for the prevention
of morbidity and mortality following hypoxic-ischaemic
encephalopathy in newborn infants. Cochrane Database Syst
Rev. 2020;8: CD013202.
11. Thayyil S, Pant S, Montaldo P, et al.
Hypothermia for moderate or severe neonatal encephalopathy
in low-income and middle-income countries (HELIX): a
randomised controlled trial in India, Sri Lanka, and
Bangladesh. Lancet Glob Health. 2021;9: e1273-85.
12. Thayyil S, Oliveira V, Lally PJ, et
al. Hypothermia for encephalopathy in low and middle-income
countries (HELIX): study protocol for a randomised
controlled trial. Trials. 2017; 18:432.
13. Zou R, Xiong T, Zhang L, et al.
Proton magnetic resonance spectroscopy biomarkers in
neonates with hypoxic-ischemic encephalopathy: a systematic
review and meta-analysis. Front Neurol. 2018; 9:732.
14. Ouwehand S, Smidt LC, Dudink J, et
al. Predictors of out-comes in hypoxic-ischemic
encephalopathy following hypo- thermia: a meta-analysis.
Neonatology. 2020; 117: 411-27.
15. Del Río R, Ochoa C, Alarcon A, et al.
Amplitude integrated electroencephalogram as a prognostic
tool in neonates with hypoxic-ischemic encephalopathy: a
systematic review. PLoS One. 2016;11: e0165744.
16. Jacobs SE, Berg M, Hunt R, et al.
Cooling for newborns with hypoxic ischaemic encephalopathy.
Cochrane Database Syst Rev. 2013;2013:CD003311.
17. Abate BB, Bimerew M, Gebremichael B,
et al. Effects of therapeutic hypothermia on death among
asphyxiated neonates with hypoxic-ischemic encephalopathy: A
systematic review and meta-analysis of randomized control
trials. PLoS One. 2021;16: e0247229.
18. Catherine RC, Ballambattu VB,
Adhisivam B, et al. Effect of therapeutic hypothermia on the
outcome in term neonates with hypoxic ischemic
encephalopathy-a randomized controlled trial. J Trop Pediatr.
2021 Jan 29;67: fmaa073.
19. Aker K, Støen R, Eikenes L, et al.
Therapeutic hypothermia for neonatal hypoxic-ischaemic
encephalo-pathy in India (THIN study): a randomised
controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;
105:405-411.
20. Tanigasalam V, Bhat V, Adhisivam B,
et al. Does thera-peutic hypothermia reduce acute kidney
injury among term neonates with perinatal asphyxia? – a
randomized controlled trial. J Matern Fetal Neo Med. 2016;
29: 2545-48.
21. Rakesh K, Vishnu Bhat B, Adhisivam B,
et al. Effect of therapeutic hypothermia on myocardial
dysfunction in term neonates with perinatal asphyxia – a
randomized controlled trial. J Matern Fetal Neonatal Med.
2018; 31: 2418-23.
22. Chen X, Peng W, Zhang Z, et al.
Efficacy and safety of selective brain hypothermia therapy
on neonatal hypoxic-ischemic encephalopathy [Chinese].
Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018; 30:1046-50.
23. Bonifacio SL, McDonald SA, Chock VY,
et al. Differences in patient characteristics and care
practices between two trials of therapeutic hypothermia.
Pediatr Res. 2019; 85:1008-15.
24. Pauliah SS, Shankaran S, Wade A, et
al. Therapeutic hypothermia for neonatal encephalopathy in
low- and middle-income countries: a systematic review and
meta-analysis. PLoS One. 2013;8:e58834.
25. Campbell H, Eddama O, Azzopardi D, et
al. Hypothermia for perinatal asphyxia: trial-based quality
of life at 6-7 years. Arch Dis Child. 2018; 103:654-659.
26. Rivero-Arias O, Eddama O, Azzopardi
D, et al. Hypo-thermia for perinatal asphyxia: trial-based
resource use and costs at 6-7 years. Arch Dis Child Fetal
Neonatal Ed. 2019;104:F285-92.
27. Shankaran S, Laptook AR, Pappas A, et
al. Effect of depth and duration of cooling on death or
disability at age 18 months among neonates with
hypoxic-ischemic encephalo-pathy: a randomized clinical
trial. JAMA. 2017; 318:57-67.
28. Shankaran S, Laptook AR, Pappas A, et
al. Effect of depth and duration of cooling on deaths in the
NICU among neonates with hypoxic ischemic encephalopathy: a
rando-mized clinical trial. JAMA. 2014; 312:2629-39.
29. Saw CL, Rakshasbhuvankar A, Rao S, et
al. Current practice of therapeutic hypothermia for mild
hypoxic ischemic encephalopathy. J Child Neurol. 2019;
34:402-409.
30. Kariholu U, Montaldo P, Markati T, et
al. Therapeutic hypothermia for mild neonatal
encephalopathy: a syste-matic review and meta-analysis. Arch
Dis Child Fetal Neonatal Ed. 2020; 105:225-28.
Neonatologist’s Viewpoint
Therapeutic hypothermia (TH) is the only
intervention well-proven to improve intact survival in neonates
with moderate-severe hypoxic ischemic encephalopathy [1]. It is
the standard of care in high income countries (HICs) and is
recommended by the International Liaison Committee on
Resuscitation (ILCOR) 2020 in low- and middle-income countries
(LMICs), though it is a weak recommendation with low evidence
[2]. A recent meta-analysis of 675 infants from 7 RCTs from
LMICs showed a 50% reduction in mortality in LMICs and found
higher effect size in LMICs as compared to HICs [3]. The results
of the HELIX trial with 408 infants are in contradiction to this
and the conclusion and the commentaries by the authors have cast
a cloud on the practice of TH in LMICs [4].
The HELIX trial, an apparently well-conducted
trial with excellent follow-up rates, did not find a difference
in the primary outcome of disability-free survival at 18 months
and has recommended to stop TH in LMICs [4]. However, there are
several issues in the trial that need further clarification. The
first is the case-mix. Unlike most other hypothermia trials from
India, two-third of the infants were outborn who reached the
cooling center at a median time of >3 hours. The screening for
enrolment is unlikely to have been optimal considering that only
2296 infants were screened in 3.5 years in seven very
high-volume public health facilities, where the annual NICU
admission is often double this number. Further, the lack of an
objective risk assessment score raises concerns that the babies
in the study, particularly in the hypothermia arm, were sicker,
indicating a selection bias. The complications in pregnancy and
emergency cesarean section were higher in the hypothermia arm.
This is especially a cause for perturbation in this study, where
the authors state that "professionals showed a strong bias
towards cooling therapy" coupled with "parental decisions
that were heavily influenced by a trust in doctors to make the
right decision on their behalf " [5].
The second issue is the fidelity to the
intervention. Early initiation of cooling and the ‘time to
target tempe-rature’ is critical to improved outcomes. Cooling
beyond 6 hours has been found to be of no benefit [6]. In fact,
a recent study suggests cooling to be done before 3 hours. In
the HELIX trial, the inclusion criteria states that baby should
be "randomized" within 6 hours of birth and the mean
randomization time is mentioned; the time to target temperature
is not mentioned. Review of Fig. 1 shows that the mean
time of achieving 33.5º is 6 hours post-randomization. The mean
age at admission to the cooling unit in outborn babies who
constitute 2/3rd of the subjects being 3 hours suggests that
most infants in the intervention arm achieved the target
temperature at approximately 9 hours. This could be one major
difference from other studies that have shown benefit, where the
time to target temperature has been less than two hours [7,8].
The rate of rewarming was also 0.5
per hour as against the
currently recommended 0.25 per hour [9].
A higher proportion of babies in the
hypothermia arm were treated with inotropes,
sedatives/analgesics and antibiotics [4]. Assessment of shock is
a challenge during therapeutic hypothermia [10], and it is
plausible that medications are confounders in this study.
The next issue is the high mortality in both
arms. Of the seven centers, five centers that contributed >90%
of the subjects had high regional neonatal mortality. High
regional neonatal mortality, which is a reflection of the
quality of care coupled with the learning curve of a new
intervention, may not permit the true benefits of an
intervention to surface. This is in contrast to Indian RCTs that
have reported low mortality (1.7-28%) during TH [3].
Characteristics of the HELIX hospitals with quality-of-care
measures such as survival rates, shared use of thermal control
device and infection control rates in the supple-mentary
appendix would have helped understand the generalizability of
the study. The wide variation in survival in centers across
India that has been highlighted in other collaborative studies
[11] and the results of the HELIX trial cannot be extrapolated
to centers with low mortality rates.
It is worth noting note that the primary
composite outcome of death or disability in hypothermia arm was
similar to the control arm despite higher mortality, suggesting
that there is indeed some benefit of hypother-mia in preventing
brain damage [4]. Severe disability among survivors were halved
and disabling cerebral palsy was reduced by 47% [11% vs 21%; RR
(95% CI) 0.53 (0.28-0.98)].
Considering the above issues, the sweeping
recommendation of the authors not to offer TH in all tertiary
care intensive care facilities is unfounded on evidence.
However, what the HELIX trial has shown that it is not the time
for all NICUs to embrace TH without setting the infrastructure,
resources and quality care for safe implementation of TH. With
the high burden of asphyxia-related mortality and morbidity, we
need to explore and study how to make TH safe in LMICs.
Collaborative efforts by hospitals that have low mortality with
cooling therapy, constant vigil, a national database,
benchmarking and efforts to get outborn babies early to cooling
hospitals that have shown good outcomes are some of the steps
way forward. I feel that it is certainly not the time to write
the epitaph on cooling in LMICs.
Funding: None; Competing interests:
Participated as faculty in therapeutic hypothermia workshops
organized by MiraCradle.
Suman Rao PN
Department of Neonatology,
St. John’s Medical College Hospital,
Bangalore,Karnataka.
raosumanv@gmail.com
References
1. Papile LA, Baley JE, Benitz W, et al.
Committee on Fetus and Newborn. Hypothermia and Neonatal
Encephalo-pathy. Pediatrics. 2014;133:1146-50.
2. Wyckoff MH, Wyllie J, Aziz K, et al.
Neonatal Life Support Collaborators. Neonatal Life Support: 2020
International Consensus on Cardiopulmonary Resusci-tation and
Emergency Cardiovascular Care Science with Treatment
Recommendations. Circulation. 2020;142: S185-221.
3. Abate BB, Bimerew M, Gebremichael B, et
al. Effects of therapeutic hypothermia on death among
asphyxiated neonates with hypoxic ischemic encephalopathy: A
systematic review and meta-analysis of randomized control
trials. PLoS One. 2021;16: e0247229.
4. Thayyil S, Pant S, Montaldo P, et al.
Hypothermia for moderate or severe neonatal encephalopathy in
low-income and middle-income countries (HELIX): a randomised
controlled trial in India, Sri Lanka, and Bangladesh. Lancet
Glob Health. 2021;9:e1273-85.
5. Pant S, Elias MA, Woolfall K, et al. HELIX
Trial consortium investigators. Parental and professional
perceptions of informed consent and participation in a
time-critical neonatal trial: a mixed-methods study in India,
Sri Lanka and Bangladesh. BMJ Glob Health. 2021; 6:e005757.
6. Natarajan G, Laptook A, Shankaran S.
Therapeutic hypothermia: How can we optimize this therapy to
further improve outcomes? Clin Perinatol. 2018 ;45:241-255.
7. Shankaran S, Laptook AR, Ehrenkranz RA, et
al. National Institute of Child Health and Human Development
Neonatal Research Network. Whole-body hypothermia for neonates
with hypoxic-ischemic encephalopathy. N Engl J Med.
2005;353:1574-84.
8. Azzopardi DV, Strohm B, Edwards AD, et al.
TOBY Study Group. Moderate hypothermia to treat perinatal
asphyxial encephalopathy. N Engl J Med. 2009;361:1349-58.
9. Clinical guidelines (Nursing) Therapeutic
hypothermia in the neonate. Available from:https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Therapeutic_
hypothermia_in_the_neonate
10. Habib S, Saini J, Amendoeira S, et al.
Hemodynamic instability in hypoxic ischemic encephalopathy: More
than just brain injury-understanding physiology, assessment, and
management. Neonatal Netw. 2020;39:129-36.
11. Murki S, Kumar N, Chawla D, et al; VLBW
Infant Survival in Hospitals of India (VISHI) Study
Investigators. Variability in survival of very low birth weight
neonates in hospitals of India. Indian J Pediatr.
2015;82:565-67.
Pediatric Neurologist’s Viewpoint
Protection of the developing brain had been
the holy grail of neonatal practice over the years. The
important goals of early and accurate identification of the
insults to the fetal and neonatal brain, understanding the
complex neurobiology of these insults and developing appropriate
mitigation strategies still remain elusive. The utility of
classical clinical approach is often very minimal in the newborn
in view of the limited repertoire of neurological signs and
symptoms [1].
Early identification and stratification of
the insults to the immature brain, based on the potential for
future neurodevelopmental disabilities, will help the clinicians
predict the clinical and developmental outcomes much more
accurately. Families too can take better learned decisions
regarding the continuation of life support in the NICU. It will
also help the research community to develop better targeted
acute interventions for the really vulnerable babies improving
the benefit: risk ratio. However, the current understanding of
neonatal neurology is far from satisfactory to make such
accurate assumptions. Some general categorizations are possible
based on the clinical data and investigations.
Neonatal encephalopathy at term with
documented evidence for intrapartum sentinel hypoxic/ischemic
events is possibly such a group. This cohort is usually much
more homogenous in developed countries, where there are robust
protocols for antenatal care and intrapartum monitoring. In
populations with poor maternal health status, antenatal care and
intrapartum monitoring, the clinical syndrome of neonatal
encephalopathy might be the composite end result of multiple on
going and one-time insults to the developing brain occurring
throughout the antenatal and perinatal periods. Without reliable
bio-markers, either imaging or biochemical, it will be difficult
to stratify this cohort into much more homogenous groups.
The story of neuroprotective interventions
for majority of the acquired brain insults has not been very
encouraging till now. Most of the proposed ones fell by the
wayside while moving from bench to the bedside, mainly due to
the undesirable side effects or lack of the predicted clinical
benefits [2]. However, TH for moderate/severe hypoxic ischemic
encephalopathy in term newborn babies has shown to be
consistently effective in reducing long term disabilities in
several well-conducted trials and is currently considered the
standard of care in most of the developed world [3,4]. TH has
also shown to be effective in reducing the burden of neonatal
seizures in this group [5].
The recently published HELIX trial [6] – a
randomized controlled trial conducted in a few large public
hospitals in South Asia, has raised major safety concerns for
therapeutic hypothermia in LMICs. The HELIX trial data suggested
that therapeutic hypothermia alongside optimal tertiary neonatal
intensive care significantly increased the incidence of death
relative to a control group without any reduction in brain
injury on MRI or improvement in the combined outcomes of death
or disability after neonatal encephalopathy [6]. There are two
very important aspects here – lack of efficacy and potential for
harm. The latter has much more serious implications, in view of
the potentially higher risk of occurrence in routine clinical
practice compared to the controlled settings of a randomized
trial.
Why did the HELIX trial show a potential for
serious harm? Such a serious safety signal was not apparent in
any of the previous studies conducted in the developed world.
The reasons might be neurobiological as the authors are trying
to argue. The clinical syndrome of neonatal encephalopathy in
LMICs might represent a totally different cohort compared to the
developed world for the reasons described above. Moreover, there
might be some inherent genetic variations affecting the
suscepti-bility to hypoxic ischemic injury as well as response
to cooling in this population. The pragmatic design and
processes used in this trial, developed probably to suit the
already existing practices in the study centers [6], might also
have contributed to this outcome. However, one factor clearly
emerging out of this well-conducted study is that safety margins
are very narrow for the current practice of therapeutic
hypothermia for neonatal encephalopathy. The tendency to offer
this intervention across all settings might result in
considerable harm, especially in the LMICs.
What’s the way forward? We can look at the
HELIX data more closely to identify any potential subgroups with
higher or lower safety margins compared to the total cohort.
Such an analysis might possibly give us more insights into the
complex neurobiology of neonatal encephalopathy/ therapeutic
hypothermia and might also help us modify the current clinical
care protocols. It might also lead to further studies to
identify new biomarkers and to explore better preventive and
interventional strategies for neonatal encephalopathy. There is
an urgent need to set up large prospective multicentric neonatal
brain consortiums in the country with standardized protocols for
clinical care, data capture and outcome analysis. Such an
approach might possibly help us stratify neonatal encephalopathy
into more homogenous groups for better targeted interventions.
Funding: None; Competing interests:
None stated.
KP Vinayan
Department of Pediatric Neurology,
Amrita Institute of Medical Sciences, Cochin.
vinayankp@aims.amrita.edu
References
1. Pressler RM, Cilio MR, Mizrahi EM, et al.
The ILAE classification of seizures and the epilepsies:
Modification for seizures in the neonate. Position Paper by the
ILAE Task Force on Neonatal Seizures. Epilepsia. 2021;62:615-28.
2. Faden A I, Stoica B. Neuroprotection:
challenges and opportunities. Arch Neurol. 2007;64:794-800.
3. Shankaran S, Laptook AR, Ehrenkranz RA, et
al. Whole-body hypothermia for neonates with hypoxic-ischemic
encephalopathy. N Engl J Med. 2005; 353: 1574-84
4. Jacobs SE, Berg M, Hunt R, et al. Cooling
for newborns with hypoxic ischaemic encephalopathy. Cochrane
Database Sys Rev. 2013:CD003311.
5. Gano D, Orbach SA, Bonifacio SL, et al.
Neonatal seizures and therapeutic hypothermia for
hypoxic-ischemic encephalopathy. Mol Cell Epilepsy. 2014; 1:
e88.
6. Thayyil S, Pant S, Montaldo P, et al. Hypothermia for
moderate or severe neonatal encephalopathy in low and
middle–income countries (HELIX): a randomized control trial in
India, Sri Lanka and Bangladesh. Lancet Glob Health. 2021; 9:
e1273-285.

