|
|
|
Indian Pediatr 2021;58:973-977 |
 |
Gastric Lavage for Prevention of Feeding
Intolerance in Neonates Delivered Through Meconium-Stained
Amniotic Fluid: A Systematic Review and Meta-Analysis
|
|
Poonam Singh 1, Manish
Kumar2, Sriparna Basu1
From 1Department of Neonatology, All India Institute of Medical
Sciences, Rishikesh; 2Department of Pediatrics, All India Institute of
Medical Sciences, Gorakhpur, Uttar Pradesh.
Correspondence to: Dr Sriparna Basu, Professor, Department of
Neonatology, All India Institute of Medical Sciences, Rishikesh,
Uttarakhand 249203, India.
[email protected]
PROSPERO Registration Number: CRD42020159723
Published online: April 17, 2021;
PII: S097475591600310
|
Background: The role of gastric lavage in
neonates delivered through meconium-stained amniotic fluid remains
unclear.
Objective: This study evaluated the effects of
gastric lavage, compared to no gastric lavage, on the incidences of
feeding intolerance, respiratory distress, meconium aspiration synd-rome,
time to establish breastfeeding, hospitalization and pro-cedure-related
complications in late-preterm and term neonates delivered through
meconium-stained amniotic fluid.
Design: Systematic review and meta-analysis.
Data sources and selection criteria: MEDLINE,
EMBASE, CENTRAL, and other databases were searched for randomized
controlled trials and quasi-randomized controlled trials using search
terms: neonate OR newborn infant, meconium OR meconium-stained amniotic
fluid, and lavage OR gastric lavage from inception to May 2020. Data
were pooled in RevMan and analyzed in GRADE.
Results: Pooled effects (9 randomized controlled
trials, number=3668), showed a significant reduction in the incidence of
feeding intolerance (relative risk 0.70; 95% confidence interval
0.58,0.85, I2=0) after gastric lavage. No difference was observed for
the incidence of meconium aspiration syndrome (4 studies) or
procedure-related complications (7 studies). Only one study, reporting
the proportion of neonates with low oxygenation (SpO2<85%), did not find
any significant difference. No study evaluated the effects of gastric
lavage on respiratory distress, breastfeeding, and hospitalization.
Conclusions: Low-quality evidence supported the
role of gastric lavage for the prevention of feeding intolerance in
late-preterm and term neonates born through meconium-stained amniotic
fluid. Applicability of results was limited by the high risk of bias.
Well-conducted randomized controlled trials with impor-tant patient
outcomes are needed before recommending the practice of gastric lavage.
Keywords: Feeding intolerance, Gastric lavage, Meconium-stained
amniotic fluid, Neonate
|
|
M
econium-stained
amniotic fluid, complicating 9-12% of all deliveries [1,2], may
be associated with recurrent vomiting and
feeding intolerance, due to meconium-induced chemical gastritis
[3], which may delay the establishment of oral feeding resulting
in a risk of hypoglycemia, need for parenteral fluid therapy [4]
and a possibility of secondary meconium aspiration [5]. Though a
quasi-randomized study showed the benefit of gastric lavage
performed in the delivery room in reducing feeding intolerance
[6], later randomized controlled trials (RCTs) failed to
document benefit [5,7-14]. Though oro/nasogastric feeding tube
placement and gastric lavage are apparently simple procedures,
complications such as feeding tube placement errors, oxygen
desaturation, bradycardia, gastric, and esophageal perforation,
are often reported [15-19]. A previous meta-analysis in 2015 [4]
included 6 studies and found limited evidence to favor gastric
lavage to reduce the incidence of feeding intolerance in infants
delivered through meconium-stained amniotic fluid. This
systematic review and meta-analysis intended to identify,
appraise, and synthesize available evidence regarding the
efficacy and safety of gastric lavage after initial delivery
room stabilization, to prevent feeding intolerance, among
neonates delivered through meconium-stained liquor.
METHODS
The protocol for this systematic review was
registered in the International Prospective Register of
systematic Reviews (PROSPERO) database. This systematic review
was conduc-ted and reported as per Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20].
Search eligibility and search strategy:
The review included RCTs and quasi-RCTs comparing the effect of
prophylactic gastric lavage with normal saline versus no lavage
after initial stabilization at delivery room, before initiation
of feeding, in late-preterm and term neonates delivered through
meconium-stained amniotic fluid on the prevention of feeding
intolerance. Feeding intole-rance was defined as gastric residue
³30%
of the previous feed, and/or regurgitation, abdominal
distension, emesis/retching [21]. The primary outcome was the
incidence of feeding intolerance, secondary outcomes being
inci-dences of respiratory distress and meconium aspiration
syndrome, need and duration of respiratory support, time to
establish breastfeeding, exclusive breastfeeding rate at
discharge, duration of hospital stay, and the adverse effects of
gastric lavage Crossover trials, non-English publications, and
conference abstracts were excluded.
All authors independently searched the
databases MEDLINE, EMBASE, Cochrane Central Register of
Controlled Trials (CENTRAL), Cumulative Index to Nursing and
Allied Health Literature (CINAHL), Google Scholar, Scopus, and
Web of Science, from inception to May, 2020. Details of search
words and search results are given in
Web Table I. The
references of full text articles were checked for possible
inclusion as additional articles.
Data extraction and quality assessment:
After removing duplicates, individual study details were
extracted in a pre-designed format by two authors (PS, MS)
indepen-dently, including author, year of publication,
geographic location, study period, research design, sample size
(calculated and analysed total and in each group), inclusion and
exclusion criteria, procedure details, characteristics of
participants, including mean gestation, birth weight, gender,
thick/thin meconium, definitions and incidences of outcomes. Any
disagree-ment related to collated data was resolved by the third
author (SB).
Quality of studies was assessed independently
by all authors using Cochrane Collaborations Risk of Bias tool
[22] based on the domains: random sequence generation;
allocation concealment; blinding of participants and personnel;
blinding of outcome assessment; incomplete outcome data;
selective outcome reporting; other bias. Any disagreement was
resolved by mutual discussion.
Statistical analysis: Statistical
analysis was performed using Review Manager Version 5.3 [23].
Relative risk (RR) with 95% confidence interval (CI) was
calculated for all primary and secondary outcomes. Risk
difference (RD) and number needed to benefit/harm were also
calcu-lated. Heterogeneity was assessed using I 2
statistics. A fixed or random-effects model was used based on
heterogeneity. Random-effects model was used where heterogeneity
was more than 50%. Sub-group analysis was done in possible areas
of heterogeneity such as consistency of meconium,
vigorous/non-vigorous, need of positive pressure ventilation
(PPV), and the postnatal age of performing gastric lavage.
Grading of Recommen-dations Assessment, Development, and
Evaluation (GRADE) approach [24] was applied to assess the
quality of evidence for the predefined outcomes.
RESULTS
Out of the initial database search of 374
articles and 3 additional articles through manual search, 12
articles were retrieved, after screening titles, abstracts and
removing duplicates. Of these, 9 studies, including 3668
neonates [5-13], met our inclusion criteria and were subjected
to meta-analysis (Fig.1). The characteristics of the
studies included in this review are summarized in
Web Table I.
Seven trials were conducted in India [5,7-10,12,13], and two
were conducted in Saudi Arabia [6] and Nepal [11]. Two studies
were quasi-randomized [6,10], while the rest were RCTs. The
study population was homogenous across the studies. All studies
included vigorous late-preterm and term neonates born through
MSAF, who did not develop respiratory distress at birth.
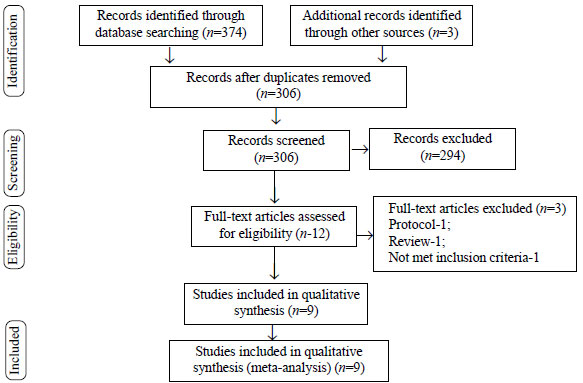 |
|
Fig. 1 PRISMA flow diagram.
|
The definition of feeding intolerance varied
across the studies. While five studies [5,7,10-12] defined
feeding intolerance as vomiting, abdominal distension, and
increased gastric residuals, two studies [6,9] considered
vomiting and retching and one study took vomiting and abdominal
distension as a signs of feeding intolerance [13]. Singh, et al.
did not mention the criteria of feeding intolerance in their
trial [8]. Very slow feeding/poor suck was considered a
component of feeding intolerance in the study of Narchi, et al.
[6]. Period of observation for feeding intolerance ranged from
48-72 hours [5,7-12] or till discharge [7,9] whereas it was not
specified by Narchi, et al. [6] and Yadav, et al. [13].
The procedure of gastric lavage varied across
the studies, using feeding tubes of variable sizes, 6 Fr
[10-12], 8 Fr [7,9,10,13] or 10 Fr [5] through oral [5,9] or
nasal route [7,10-13] and lavage being conducted using 10 mL/kg
[5,7,10-13] or 20 mL [9] of normal saline [5,7,9-13] in the
aliquots of 5 mL [10] or 10 mL [7,11,12]. Feeding intolerance
was the primary outcome in seven studies [6,7,9-13], while
proportion of infants developing meconium aspiration syndrome
within 72 hours of age [5] and the need for subsequent gastric
lavage [8], respectively, were the primary outcomes in the other
two studies. Only one study monitored the procedure of gastric
lavage using a pulse oximeter [5].
Web Fig. 1A and
Web
Fig. 1B summarize the quality of the studies. There
was no publication bias as per the Funnel plot (Web Fig.2).
Gastric lavage resulted in a significant
reduction of feeding intolerance (pooled RR 0.70; 95% CI 0.58 to
0.85, I 2 0%), with a
risk difference of -3.39% (95% CI -5.34 to -1.44), and number
needed to benefit being 29.5 (95% CI 18.69 to 69.83) (Fig. 2).
Subgroup analysis of two trials [6,7] in neonates delivered
through thick meconium-stained amniotic fluid did not find any
significant difference, though pooling of data could not be done
due to incomplete reporting. Sensitivity analysis performed
after the inclusion of only RCTs [5,7-9,11-13] and those with a
uniform definition of feeding intolerance [5,7,10-12] also
showed significant beneficial effects of gastric lavage (Web
Table II).
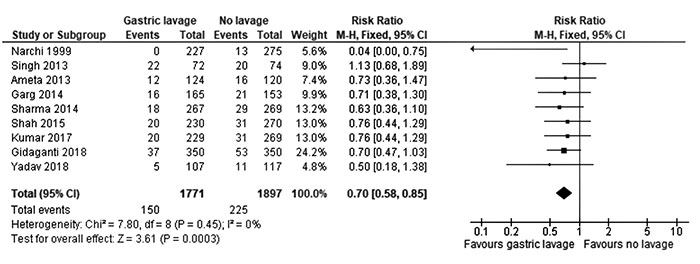 |
|
Fig. 2 Forest plot analyzing the
incidence of feeding intolerance in neonates with and
without gastric lavage.
|
While none of the neonates in any group
developed meconium aspiration syndrome in three studies
[8,9,13], one study [5] reported insignificant difference in the
incidence of meconium aspiration syndrome between gastric lavage
and no-gastric lavage group. No difference was observed across
the studies in the incidences of bradycardia [5,7,9-13],
desaturation/cyanosis [5,7,11,12] or local trauma [5-7,9-13],
between neonates with and without gastric lavage. One study [5]
reported no significant difference in the proportion of neonates
with low oxygenation (SpO2<85%
at 15 minutes of life) between the groups. None of the studies
reported complications like secondary vomiting, aspiration,
respiratory distress [6,7,9,10] or apnea [5-7, 9-13].
The time of establishment of breastfeeding,
exclusive breastfeeding rate at discharge, need and duration of
respiratory support, as well as complications like feeding tube
placement errors and gastric/esophageal perforation were not
reported by any of the studies.
The quality of evidence assessed using the
GRADE (Web Table III), shows that except for the
incidence of feeding intolerance, the effect size of other
outcomes was non-estimable due to the small number of
occurrences.
DISCUSSION
In the present systematic review, low-quality
evidence from nine RCTs showed that gastric lavage performed
immediately after delivery room stabilization before initiation
of feeding resulted in a significant reduction in the incidence
of feeding intolerance in vigorous late preterm and term
neonates born through meconium-stained amniotic fluid. None of
the studies reported any adverse events related to gastric
lavage and none of the studies looked for feeding tube placement
errors and gastric/esophageal perforation.
The studies included in this review had high
rates of bias, mainly attributable to lack of allocation
conceal-ment, quasi-randomized design [6,10] and absence of
blinding of the outcome assessors [5-13]. Seven studies
evaluated the adverse effects of gastric lavage in the form of
apnea, bradycardia, local trauma or cyanosis and no difference
was found between the groups [5,7,9-13]. Narchi, et al. [6]
assessed for apnea and local trauma, which was not documented in
any study neonate. Though all the studies reported gastric
lavage to be a safe procedure, desaturations were not evaluated
with pulse oximeters, except in one study [5]. The route of
feeding tube placement being non-uniform across studies, it
could affect the incidence of adverse effects [25]. Gastric
aspiration with feeding tube placement has been shown to
increase the mean arterial blood pressure, retching, disruption
of pre-feeding behavior [26], with the development of functional
gastrointestinal disorders later in life [27].
None of the studies have mentioned the time
of occurrence of vomiting/retching in relation to the procedure
or the initiations of feeds. The definition of feeding
intolerance was not uniform across the studies. Slow/poor
sucking, taken as feeding intolerance, by a study is more
suggestive of neurological problems or immaturity. None of the
studies have evaluated the effect of the intervention on
clinically more relevant outcomes such as time to establish
breastfeeding, exclusive breastfeeding rate at discharge, or
initiation of immediate skin-to-skin contact in the delivery
room.
Gastric lavage can potentially affect the
incidence of meconium aspiration syndrome. While on one hand,
gastric lavage may prevent meconium aspiration syndrome by
clearing meconium from the stomach, thereby preventing
subsequent vomiting and aspiration [3]; on the other hand, it
may predispose to meconium aspiration syndrome by inducing
retching, vomiting and aspiration of gastric content while
inserting the feeding tube [28]. The incidence of meconium
aspiration syndrome, reported by four studies, was low
[5,8,9,13], which could be attributed to the inclusion of only
vigorous neonates with neonates at risk for meconium aspiration
syndrome, like those with low Apgar scores, respiratory
depression requiring resuscitation were excluded.
The limitation of the review was that data
for out-comes like meconium aspiration syndrome and adverse
events could not be pooled as the reported incidences were nil
in most of the studies. Proposed subgroup ana-lyses could not be
done due to the lack of data in the included trials.
Non-vigorous neonates requiring delivery room resuscitation were
excluded by all.
To conclude, low-quality evidence supported
the role of gastric lavage for the prevention of feeding
intolerance in vigorous late preterm and term neonates born
through meconium-stained amniotic fluid. Though the procedure
seems to be apparently safe, one should be cautious to recommend
this practice as the adverse events related to gastric lavage
were not evaluated critically and the effects of this procedure
on the routine newborn care practices such as skin-to-skin
contact, and breastfeeding rates were lacking. Evidence in
non-vigorous neonates, who are more prone for the development of
respiratory distress and feeding intolerance, were lacking.
Well-designed RCTs with defined outcome variables under strict
monitoring for procedure-related complications are needed.
Note: Additional material related
to this study is available with the online version at
www.indianpediatrics.net
Contributors: PS: conceptualized the
review, literature search, data analysis and manuscript writing;
MK: literature search, data analysis and manuscript writing; SB:
conceptualized the review, literature search, data analysis and
manuscript writing.
Funding: None; Competing interest:
None stated.
REFERENCES
1. Viraraghavan VR, Nangia S, Prathik BH,
et al. Yield of meconium in non-vigorous neonates undergoing
endotracheal suctioning and profile of all neonates born
through meconium-stained amniotic fluid: A prospective
observational study. Paediatr Int Child Health.
2018;38:266-70.
2. Chiruvolu A, Miklis KK, Chen E, Petrey
B, Desai S. Delivery room management of meconium-stained
newborns and respiratory support. Pediatrics.
2018;142:e20181485.
3. Narchi H, Kulaylat N. Feeding problems
with the first feed in neonates with meconium-stained
amniotic fluid. Paediatr Child Health. 1999;4:327 30.
4. Deshmukh M, Balasubramanian H, Rao S,
Patole S. Effect of gastric lavage on feeding in neonates
born through meconium-stained liquor: A systematic
review. Arch Dis Child Fetal Neonatal Ed. 2015;100:F394-9.
5. Gidaganti S, Faridi MM, Narang M,
Batra P. Effect of gastric lavage on meconium aspiration
syndrome and feed intolerance in vigorous infants born with
meconium stained amniotic fluid - A randomized control
trial. Indian Pediatr. 2018;55:206-10.
6. Narchi H, Kulaylat N. Is gastric
lavage needed in neonates with meconium-stained amniotic
fluid? Eur J Pediatr. 1999;158:315-7.
7. Ameta G, Upadhyay A, Gothwal S, Singh
K, Dubey K, Gupta A. Role of gastric lavage in vigorous
neonates born with meconium stained amniotic fluid. Indian J
Pediatr. 2013;80:195 8.
8. Singh KB, Jain R, Babu R, Jyoti J,
Singh MK, Parasher I. Role of routine gastric lavage in term
and late preterm neonates born through meconium stained
amniotic: a randomised control trial. J Evol Med Dent Sci.
2013;2:9868-75.
9. Sharma P, Nangia S, Tiwari S, Goel A,
Singla B, Saili A. Gastric lavage for prevention of feeding
problems in neonates with meconium-stained amniotic fluid: A
randomised controlled trial. Paediatr Int Child Health.
2014;34:115 9.
10. Garg J, Masand R, Tomar BS. Utility
of gastric lavage in vigorous neonates delivered with
meconium stained liquor: a randomized controlled trial. Int
J Pediatr. 2014;2014:204807.
11. Shah L, Shah GS, Singh RR, Pokharel
H, Mishra OP. Status of gastric lavage in neonates born with
meconium stained amniotic fluid: A randomized controlled
trial. Ital J Pediatr. 2015;41:85.
12. Kumar A, Gupta RP, Singh A. Role of
gastric lavage in newborn with meconium stained amniotic
fluid: A randomized controlled trial. IOSR-JDMS.
2017;16:51-3.
13. Yadav SK, Venkatnarayan K, Adhikari
KM, Sinha R, Mathai SS. Gastric lavage in babies born
through meconium stained amniotic fluid in prevention of
early feed intolerance: A randomized controlled trial. J
Neonatal Perinatal Med. 2018;11:393 7.
14. Hutton EK, Thorpe J. Consequences of
meconium stained amniotic fluid: what does the evidence tell
us? Early Hum Dev. 2014;90:333 9.
15. Quandt D, Schraner T, Ulrich Bucher
H, ArlettazMieth R. Malposition of feeding tubes in
neonates: is it an issue? J Pediatr Gastroenterol Nutr.
2009;48:608-11.
16. Shiao SY, Youngblut JM, Anderson GC,
DiFiore JM, Martin RJ. Nasogastric tube placement: Effects
on breathing and sucking in very-low-birth-weight infants. Nurs
Res. 1995;44:82 8.
17. Gasparella M, Schiavon G, Bordignon
L, et al. Iatrogenic traumas by nasogastric tube in very
premature infants: our cases and literature review. Pediatr
Med Chir. 2011;33:85 8.
18. Maruyama K, Shiojima T, Koizumi T.
Sonographic detection of a malpositioned feeding tube
causing esophageal perforation in a neonate. J Clin
Ultrasound. 2003;31:108 10.
19. Metheny NA, Meert KL, Clouse RE.
Complications related to feeding tube placement. Curr Op
Gastroenterol. 2007;23:178 82.
20. Moher D, Liberati A, Tetzlaff J,
Altman DG; PRISMA Group. Preferred reporting items for
systematic reviews and meta-analyses: The PRISMA statement.
PLoS Med. 2009;6:e1000097.
21. Fanaro S. Feeding intolerance in the
preterm infant. Early Hum Dev. 2013;89:S13-20.
22. Higgins JPT, Thomas J, Chandler J, et
al, editors. Cochrane Handbook for Systematic Reviews of
Interventions. 2nd ed. Chichester (UK): John Wiley & Sons,
2019.
23. Review Manager (RevMan) [Computer
program]. Version 5.4, The Cochrane Collaboration, 2020.
24. Schünemann H, Broek J, Guyatt G,
Oxman A, editors. GRADE handbook for grading quality of
evidence and strength of recommendations. Updated October
2013. The GRADE working group, 2013.
25. Greenspan JS, Wolfson MR, Holt WJ,
Shaffer TH. Neonatal gastric intubation: differential
respiratory effects between naso-gastric and orogastric
tubes. Pediatr Pulmonol. 1990;8:254-8.
26. Widström AM, Ransjö-Arvidson AB,
Christensson K, Matthiesen AS, Winberg J, Uvnäs-Moberg K.
Gastric suction in healthy newborn infants. Effects on
circulation and developing feeding behaviour. Acta Paediatr
Scand. 1987;76:566-72.
27. Anand KJ, Runeson B, Jacobson B.
Gastric suction at birth associated with long-term risk for
functional intestinal disorders in later life. J Pediatr.
2004;144:449-54.
28. Khazardoost S, Hantoushzadeh S, Khooshideh M, Borna S.
Risk factors for meconium aspiration in meconium stained
amniotic fluid. J Obstet Gynaecol. 2007;27:577-9.
|
|
|
 |
|

