|
|
|
Indian Pediatr 2021;58:940-942 |
 |
Serum IgG Titers
Against Toxoplasma gondii in Uninfected Infants Exposed
In Utero to Toxoplasmosis
|
|
Daniela Pires Ferreira Vivacqua, Ana Cristina Cisne Frota, Mariana
Guerreiro Martins, Thalita Fernandes Abreu, Cristina Barroso Hofer
From Department of Infectious Diseases, Instituto de Puericultura e
Pediatria Martagão Gesteira, Universidade Federal do Rio de Janeiro, Rio
de Janeiro, Brazil.
Correspondence to: Daniela Pires Ferreira Vivacqua, R Bruno Lobo, 50,
Ilha do Fundão, Rio de Janeiro, Brazil.
E-mail: [email protected]
Received: April 30, 2020;
Initial review: June 02, 2020;
Accepted: May 12, 2021.
Published online: May 20, 2021;
PII:S097475591600323
|
Objective: To describe the mean time of decrease
of T. gondii IgG titers in uninfected infants exposed in utero to
toxoplasmosis. Methods: A retrospective cohort study was
conducted between 2008-2017, among infants under 12 months and exposed
in utero to toxoplasmosis. Serial monthly monitoring of serum IgG titers
were done till undetectable levels. Results: 240 infants with
mean gestational age at diagnosis of 19.2 weeks were included in the
study. The mean (range) time for IgG level to become undetectable was
7.9 (0.8-25.0) months. 14 infants became negative between 13-24 months.
Conclusion: Majority of asymptomatic infants exposed in utero to
T. gondii become seronegative before 12 months of age.
Keywords: Chorioretinitis, Intrauterine
infection, Maternal exposure, TORCH.
|
|
Toxoplasmosis is one of
most prevalent
infectious diseases in the World [1,2], with a
prevalence of 60-80% in Brazil [3].
Approximately half of infected people are asymptomatic;
however, infection during pregnancy can cause chorioretinitis
and delayed psychomotor develop-ment in infants [4,5]. A
congenital toxoplasmosis surveillance system was established in
Brazil in 2016, which estimated rates between 0.3-1.3/1000 live
births, one of the highest in the world [6,7].
Guidelines on management of infants exposed
to toxoplasmosis in utero recommend screening paired blood
samples from mother and baby and the target organs for disease
at birth [8-10]. Infants are considered not infected if
Toxoplasma gondii immunoglobulin IgM titers are negative and
IgG titers are equal or lower than their mothers, with no
evidence of congenital toxoplasmosis after complete clinical,
radiologic, and laboratory evaluation. In these exposed infants
monthly measurement of T. gondii IgG is recommended to
exclude congenital infection. Levels of T. gondii IgG
titers are expected to reduce by half every month until
undetectable [2].
The follow-up of asymptomatic exposed infants
can be time-consuming, and costly for health services and
families. The aim of this study was to observe the time of
decrease of T. gondii IgG titers of asymptomatic infants
exposed in utero to toxoplasmosis.
METHODS
This retrospective cohort study was conducted
at a reference pediatric infectious diseases center in a
tertiary pediatric hospital, University of Rio de Janeiro,
Brazil from 2008 to 2017. The study was approved by the
institutional review board.
All infants up to 12 months of age referred
with history of in utero exposure to T. gondii without
infection at the end of follow-up were included. The study
excluded subjects who were not followed up until the diagnostic
definition, those who were referred after 12 months of life,
those whose medical records were not available and those who
were diagnosed with congenital toxoplasmosis during the follow
up. The infant’s vertical exposure to T. gondii was
diagnosed by maternal acute infection during pregnancy defined
by presence of serum IgM or reactive IgG for T. gondii in
a woman with previously non-reactive IgG level. Additional
criteria to define exposure without congenital toxoplasmosis
were normal central nervous system (CNS) imaging by
ultrasonography or tomography, normal fundoscopy, negative
polymerase chain reaction test for T.gondii in amniotic
fluid, negative T. gondii IgM, and undetectable IgG
titers for T. gondii before one year of age [11]. IgA
testing was not done as it was not available. The infant was
considered as having congenital infection if any of these tests
presented evidence of toxoplasmosis infection. The laboratory
method used for the specific T. gondii serology varied
during the time of the study due to government supplies’
availability. In most of them, the IgG was considered
non-reactive if less than 1.0 IU/mL Nevertheless, every time
there was a change in methods, another serology was ordered for
the children, as soon as possible, to make sure that the titers
were decreasing.
Obstetric, clinical, demographic, and
laboratory data were obtained from the medical records and
collected in a standardized form. All data were included in a
database using Access 2016 and analyses were performed using
STATA software (version 13.0; Stata Corp LP) statistical
program. Categorical and continuous variables were described by
frequencies, central (mean and median) and dispersion measures
(IQR). The time between birth and the first non-reactive T.
gondii IgG sample was calculated and described in median
(IQR).
RESULTS
In this study, 432 medical records of
newborns and infants with a history of in utero exposure to
toxoplasmosis were collected. The selection of the participants
is shown in Fig. 1. A total of 240 exposed infants with
mean gestational age 39 weeks, and mean birth weight and head
circumference as 3231 g and 34.3 cm, respectively were included.
The mean maternal age was 24.7 years and the mean gestational
age at the time of maternal diagnosis of T. gondii
infection was 19.2 weeks (35.5% in the first and 42.7% in the
second trimester of pregnancy). Treatment with spiramycin or
sulfadiazine and pyrimethamine was performed in 76.3% of
mothers. Only 9 (3.7%) mothers reported any specific symptoms of
toxoplasmosis, one had non-specific flu-like symptoms, 78
(32.5%) were asymptomatic (diagnosed through prenatal
screening) and 152 (63.3%) did not have any information about
the symptoms.
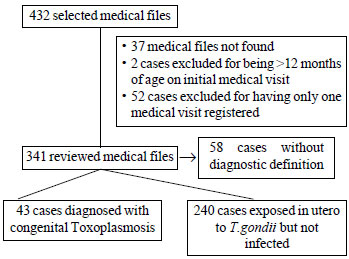 |
|
Fig. 1 Flow of the study.
|
The mean (range) time for toxoplasmosis IgG
titers to become undetectable in the serum was 7.9 (0.8 to 25.0)
months. The median (IQR) T. gondii IgG titers at the
first visit were 115 (45, 223) U/mL. Fig. 2 shows the
time span to reach undetectable/ negative IgG titers in these
patients, showing that 50% of uninfected infants took 7.3 (95%
CI: 6.83-7.76) months to have a non-reactive serology. One
infant had a positive IgM test after birth which was found to be
non-reactive when tested after day five of life. The average age
when the IgG levels became non-reactive did not change during
the study period (data not shown).
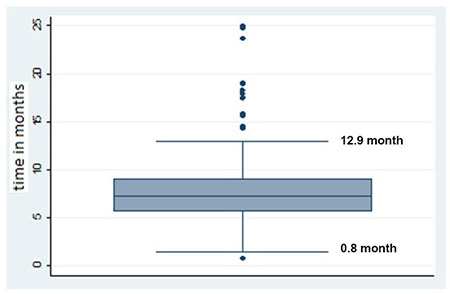 |
|
Fig. 2 Box-plot showing time
span for toxoplasmosis IgG to reach undetectable levels.
|
Fourteen infants took more than 12 months
(range 13 to 24 months), to present negative serology, 7 infants
had a gap of more than 2 months between IgG titers measures near
the 12 months mark. The remaining seven patients reached
undetectable IgG titers between 14 to 19 months and remained
asymptomatic throughout the follow-up, with normal target organ
screening tests repeated a few times and a clear monthly drop in
IgG titers.
DISCUSSION
In this study, we found that the mean age for
IgG titer to become undetectable in newborn in utero exposed but
not infected by T. gondii was 7.9 months.
A major limitation was missing data and
medical records. The follow-up required frequent visits over a
long period of time, which led some families to miss
appointments or abandon the follow-up. The lack of standardized
technique to perform T. gondii serology and change in
laboratory techniques with time was another limitation.
The Brazilian guidelines recommend additional
hematological and liver function tests in infants exposed in
utero to T. gondii [9]. T. gondii IgG maternal
antibodies passed to the newborn are expected to decrease
by 50% each month until non-reactive between 6 and 12 months of
life [2,12]. Therefore, a follow-up with monthly serological
T. gondii tests until negativity of IgG is recommended
[9,11]. Our results found a similar age range to reach
undetectable specific IgG serology of 7.9 months.
In this study, the age for IgG titers to
become undetectable ranged from 0.8 to 25.0 months unlike the
range of 6 to 12 months described earlier [2]. Therefore,
asymptomatic infants with low IgG titers should not be
classified as infants with congenital toxoplasmosis at 12
months, and the presence of reactive IgG after 12 months as a
diagnostic criterion for congenital toxoplasmosis should be
re-evaluated. Asymptomatic infants with low IgG titers should be
analyzed individually to serially monitor IgG decrease at 12
months of age. In such cases, patients may continue to be
followed and be considered as only exposed in utero to
toxoplasmosis but not infected when the serology is negative
after 12 months of life.
Ethics clearance: IPPMG institutional
review board; CAAE: 74564017.7.0000.5264, November, 2017.
Contributors: DV: data collection,
analysis and manuscript prepration; MM: data collection, writing
of the manuscript and was the responsible for its translation to
English; ACF,TA: conceptualization study and manuscript review;
CH: conceived the initial idea and the design of the study, data
analyses, manuscript review. All authors approve the final
manuscript.
Funding: None; Competing interest:
None stated.
|
WHAT THIS STUDY ADDS?
•
Asymptomatic infants
exposed in utero to toxoplasmosis may take longer than
12 months of age to achieve undetectable IgG titers.
|
REFERENCES
1. Torgerson PR, Mastroiacovo P. The
global burden of congenital toxoplasmosis: A systematic
review. Bull World Health Organ. 2013; 91:501-08.
2. Remington JS, McLeod R, Thulliez P, et
al. Infectious Disease of the Fetus and Newborn Infant, 7th
edition. Saunders, 2010.p. 947-1091.
3. Dubey JP, Lago EG, Gennari SM, Su C,
Jones JL. Toxoplasmosis in humans and animals in Brazil:
High prevalence, high burden of disease, and epidemiology.
Parasitology. 2012;139:1375-424.
4. Boyer KM, Holfels E, Roizen N, et al.
Risk factors for Toxoplasma gondii infection in mothers of
infants with congenital toxoplasmosis: Implications for
prenatal management and screening. Am J Obstet Gynecol.
2005; 192:567-71.
5. Boyer K, Hill D, Mui E, et al.
Unrecognized ingestion of Toxoplasma gondii oocysts leads to
congenital toxoplas-mosis and causes epidemics in North
America. Clin Infect Dis. 2011;53:1081-9.
6. Neto EC, Anele E, Rubim R, et al. High
prevalence of congenital toxoplasmosis in Brazil estimated
in a 3-year prospective neonatal screening study. Inter J
Epidemiol. 2000;29: 941-7.
7. Carellos EVM, Caiaffa WT, Andrade GMQ,
et al. Congenital toxoplasmosis in the state of Minas Gerais,
Brazil: A neglected infectious disease? Epidemiol Infect.
2014;142:644-55.
8. Maldonado YA, Read JS. Diagnosis,
treatment, and prevention of congenital toxoplasmosis in the
United States. Pediatrics. 2017;139: e20163860.
9. Health Care Department, Strategic
programatic actions. "Gestação de Alto Risco - Manual
Técnico" - Manual for High Risk Pregnancies, 5th ed.
Ministry of Health, Brazil, 2012.
10. Lebech M, Joynson DHM, Seitz HM, et
al. Classification system and case definitions of Toxoplasma
gondii infection in immunocompetent pregnant women and their
congeni-tally infected offspring. European Research Network
on Congenital Toxoplasmosis. Eur J Clin Microbiol Infect Dis.
1996;15:799-805.
11. Laboratory tests for the diagnosis of
toxoplasmosis. Sutter Health. Palo Alto Medical Fundation.
Accessed April 28, 2020. Available from:https://www.sutterhealth.org/
pamf/services/lab-pathology/serology-clinician-guide
12. Liwoch-Nienartowicz N, Toczylowski K, Jankowska D,
Bojkiewicz E, Oldak E, Sulik A. The rate of waning of maternal
antibodies against toxoplasma gondii in uninfected infants.
Ljubljana, Slovenia, 2019. Conference paper at the 37th
Annual Meeting of the European Society for Paediatric Infectious
Diseases, 2019.
|
|
|
 |
|

