|
|
|
Indian Pediatr 2021;58:936-939 |
 |
Comparison of the
Predictive Accuracy of Stool Color for Triage of Infants for
Phototherapy (STrIP) Score With Transcutaneous Bilirubinometer
in Predicting Serum Bilirubin in Neonates
|
|
Sushma Krishnegowda, Basil John Thomas, Deepti Thandaveshwara,
Srinivasa Murthy Doreswamy
From Department of Pediatrics, JSS Academy of Higher Education and
Research, MG Road, Mysuru, Karnataka.
Correspondence to: Dr Srinivasa Murthy Doreswamy, Professor,
Department of Paediatrics, JSS Academy of Higher Education and Research,
Mysuru, Karnataka. [email protected]
Received: July 02, 2020;
Initial review: September 28, 2020;
Accepted: May 05, 2021.
Published online: May 20, 2021;
PII:S097475591600326
|
Objectives: To compare the agreement of stool
color for triage of infants for phototherapy (STrIP) score and
transcutaneous bilirubinometer values with measured serum bilirubin in
neonatal hyperbilirubinemia. Methods: Babies more than 35 weeks
of gestation, with clinical jaundice, and on exclusive breastfeeding
were included in the study. Babies with who were clinically unstable or
who had received phototherapy based on clinical assessment were
excluded. The agreement was analyzed using Bland-Altman charts. Results
of three non-invasive methods were further compared with the measured
serum bilirubin levels. Results: There was a mean difference of 4
mg/dL of bilirubin between transcutaneous bilirubin and serum bilirubin
levels, whereas the agreement between the STrIP score and Serum
bilirubin shows a difference of only 2 mg/dL. On further analysis of
Kramer, transcutaneous and STrIP score, method of bilirubin estimation
against serum bilirubin, there was a mean difference 6 mg/dL, 4 mg/dL
and 2 mg/dL, respectively. Conclusion: STrIP score has the best
agreement with serum bilirubin in neonates compared to other
non-invasive techniques such as transcutaneous bilirubinometry and
clinical assessment using Kramer scale.
Keywords: Hyperbilirubinemia, Estimation, Outcome.
|
|
N
ewborn jaundice is a
common clinical
problem in first week of life [1]. Timely
intervention can prevent bilirubin toxicity
leading to kernicterus with long term morbidity [2-4]. Serum
bilirubin, an invasive test is the gold standard for determining
bilirubin levels.
There are various non-invasive methods to
predict the bilirubin levels, with each having its own advantage
and disadvantages. Original Kramer scale was further improvised
by the identification of five zones and its corresponding range
of serum bilirubin [5]. Although this was considered a low-cost
tool in screening the neonatal jaundice, it has limitations of
observer variation and difficulty in visually assessing the
jaundice in dark skinned babies [6].
Transcutaneous bilirubinometer are based on
the principle of absorption of light by the skin at particular
wavelength [7].
These devices were found to be inaccurate in severe
hyperbilirubinemia, preterm and babies who received phototherapy
[8,9]. Smart phone apps have also been developed using optical
techniques for detection of newborn jaundice [10]. These apps
have an added advantage of being detected at the earliest by the
parents at home.
We previously demonstrated that the stool
color in addition to clinical staging expressed as STrIP score
can accurately predict SBR [11]. This is based on physio-logical
plausibility of relation between entero-hepatic circulation,
stool color and serum bilirubin. STrIP score is the sum of
Kramer score and the matched stool color score, which predicts
serum bilirubin. Unlike TcB, stool color is neither influenced
by ambient light or skin color, and it does not need a
sophisticated optical device. In this study, our objective was
to compare the agreement of STrIP score and transcutaneous
bilirubinometer values with measured serum bilirubin in neonatal
hyper-bilirubinemia.
METHODS
This prospective study was conducted between
January and June, 2019 in the postnatal ward of JSS Hospital.
All babies more than 35 weeks of gestation, with clinical
jaundice, and on exclusively breastfeed were included in the
study. Babies who were clinically unstable or who had received
phototherapy based on clinical assessment were excluded. Babies
who did not pass stools during the observation time (10 AM to 1
PM) were not included in the study. Ethical clearance was
obtained by the institutional ethics committee. Informed consent
was obtained from the mother.
Blood sampling for serum bilirubin was done
on day 3 of life as per unit protocol or when clinically
indicated. Serum bilirubin estimation was done using the
principle of diazotization method. Clinical assessment of
jaundice was done using Kramer scale on the same day. STrIP
weightage was calculated with the stool sample. Stool sample was
collected within 3 hours of detection of clinical jaundice, and
the color of the stool was compared by the same investigator
with the stool color card (stool strip) and stool color
weightage was determined. When there was a gradient of color,
the darkest portion of the color was taken for determining the
stool color weightage as per the STrIP card which ranged from 1
to 5. Matching of stool color with the stool color strip was
done in day light between 10 AM and 1 PM beside the window.
STrIP score was then calculated by adding the stool color
weightage to clinical assessment by Kramer scale. At the same
time, average of three bilirubin readings was taken at the
forehead by transcutaneous bilirubinometer (MBJ 20, SAAG
Medicare system) in a quiet child soon after assessing the STrIP
score.
To estimate the agreement between two
non-invasive measurements with the gold standard (serum
bilirubin measurement), we defined the precision of 95%
confidence of limit of agreement to be 40% of the standard
deviation of the difference of two measurements based on our
pilot study for sample size. A sample size of 84 subjects was
calculated.
Statistical analysis: Statistical
analysis was done using Microsoft Excel 2016 and Analyse-it
version 4.3 for excel. Both the interventional measurements were
compared with the measured serum bilirubin using Bland - Altman
analysis for mean and the 95% limits of agreement along with
their confidence interval.
RESULTS
During the study period, a total of 332
newborns were admitted to the postnatal ward, of which 252 were
eligible. However, 94 were excluded as they met exclusion
criteria or got transferred to special wards and another 75 due
to non-passage of stools between the pre-decided time window.
Finally, a total of 83 (53% males) eligible neonates were
included in the study. Of these, 12% were 40 weeks and above,
15% had jaundice by 48 hours of age, and 51% between 48-96
hours. Among the neonates who had dehydration, the median (IQR)
dehydration was 3% (2%-4%) above the expected for age (Table
I). The mean difference between Kramer scale and SBR was
–3.2 mg/dL, between TcB and SBR was 1.6 mg/dL, and that between
STrIP score and serum bilirubin was 0.9 mg/dL.
Table I Baseline Characteristics of the Neonates With Hyperbilirubinemia (N = 83)
| Characteristic |
Value |
| Age (d) |
3 (2-5) |
| Gestation (wk) |
37 (36-38) |
| Pretermsa |
21 (25.3) |
| Birthweight (kg) |
2.9 (2.4-3.2) |
|
No dehydrationa
|
54 (65) |
| Kramer score |
7 (7-10) |
| STrIP score |
11 (9-13) |
|
Transcutaneous bilirubinometer value |
11.9 (9.7-12.7) |
| All values in median
(IQR) or ano. (%). STrIP- Stool color for triage of
infants for phototherapy. |
Bland-Altman analysis was done for agreement
for all the three non-invasive methods of prediction of
bilirubin against SBR. Kramer scale underestimates serum
bilirubin and 95% of the values assessed by Kramer scale lie
between +0.1 to –6.4 mg/dL of serum bilirubin. 95% limits of
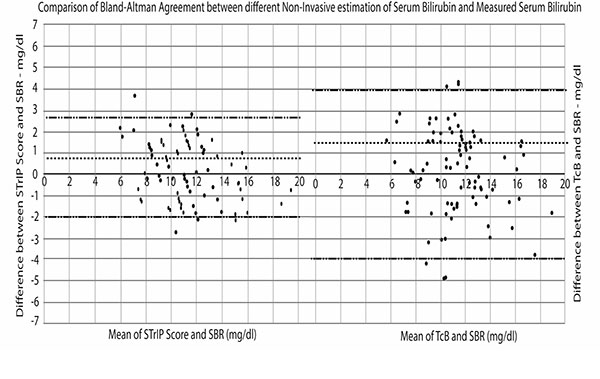
agreement between TcB, STrIP and SBR is shown in Fig.1.
TcB had a 95% limit of agreement between +3.9 to –4 mg/dL. In
contrast, the limit of agreement between STrIP and SBR was
between +2.2 and –2 mg/dL (Fig. 1).
 |
|
Fig. 1 Bland-Altman agreement between STrIP score
vs SBR and TcB vs SBR.
|
Median (IQR) TcB value and STrIP were 11.9
(9.6- 12.7) vs 11 (9-13) mg/dL (P= 0.82). The median
(IQR) SBR was 11.2 (9.1-12.6) mg/dL.
DISCUSSION
The present study demonstrates that the STrIP
score can predict serum bilirubin more accurately than the other
non-invasive method like transcutaneous bilirubino-meter, at
three days of age. The mean difference between STrIP score and
serum bilirubin was 0.9 mg/dL, much lesser as compared to other
non-invasive methods.
The visual assessment using the modified
Kramer score is the most widely practiced method of jaundice
screening among neonates. Joan, et al. [12] demonstrated Kramer
scale to be ineffective in screening jaundice with the
sensitivity and specificity being 89% and 54%, respec-tively.
Factors like skin color, birth weight, observer difference
(nurse, treating physician) are factors contributing for its
poor accuracy [13-15]. This further reinforces that the clinical
estimate by Kramer scale alone is not sufficient to detect
jaundice needing treatment. Transcutaneous bilirubinometer is
another non-invasive method used in bilirubin assessment of
newborn. Studies have concluded TcB to have better correlation
with serum bilirubin levels than visual assessment [6,15].
Although transcutaneous bilirubin assessment was found to be
better than the visual assessment of jaundice, bilirubin values
were found to be inconsistent in different sites.
Our study has shown that the values of both
STrIP score and TcB are very close to measured serum bilirubin
and there was no statistically significant difference between
them. Hence the comparison of agreement between STrIP score and
TcB values against SBR serves as clinically useful information.
The STrIP score values hover around +/- 2 mg/dL of SBR compared
to TcB which hovers around +/- 4 mg/dL. The clinical implication
of this wide variation with TcB is significant, as the margin of
error in making management decision in neonatal
hyper-bilirubinemia is very small. Among the three non-invasive
clinical estimation of bilirubin, the close match with SBR is
the STrIP score, throughout the range of clinical utility i.e.,
between serum bilirubin values of 5-20 mg/dL.
Modified Kramer scale and transcutaneous
bilirubin method was developed with the intention of having a
test which is simple, reliable, accurate and to avoid repeated
blood sampling. STrIP score has all the benefits as above along
with added advantage of promising predictive accuracy.
The limitation in this study is the possible
bias of the observer as the same person had performed TcB
measure-ment and STRiP score assessment.
To conclude, STrIP score, a simple, bedside,
easy to use, reliable non-invasive method has the best agreement
with serum bilirubin in neonates compared to other non-invasive
techniques - transcutaneous bilirubinometry and clinical
assessment using Kramer scale. Larger studies might further help
in ascertaining its utilization at the community level in early
detection of hyperbilirubinemia requiring phototherapy. More
studies are needed to conclude the same for reliable use in
larger population.
Ethics clearance: Institutional ethics
committee, JSS Medical College; No. JSSMC/IEC/
140120/19NCT/2020-21, dated 30 January, 2020.
Contributors: SK: study design, analyzing
the data and preparing the manuscript; BJT: collected the data,
did the literature search and contributed for preparation of the
manuscript; DT: has helped in literature search, and manuscript
preparation; SM: analysis of data, conceived the research
question contributed to the study design, and helped in
preparing the manuscript. All the authors have approved the
manuscript in the present form.
Funding: None; Competing interest:
None stated.
|
WHAT THIS STUDY ADDS?
• In neonates with clinical
jaundice, STrIP score had a better agreement with serum
bilirubin than transcutaneous bilirubinometer.
|
REFERENCES
1. Bhutani VK, Zipursky A, Blencowe H, et
al. Neonatal hyperbilirubinemia and rhesus disease of the
newborn: Incidence and impairment estimates for 2010 at
regional and global levels. Pediatr Res. 2013;74:86-100.
2. Ullah S, Rahman K, Hedayati M.
Hyperbilirubinemia in neonates: Types, causes, clinical
examinations, preventive measures and treatments: A
narrative review article. Iran J Public Health.
2016;45:558-68.
3. Das S, van Landeghem FKH.
Clinicopathological spectrum of bilirubin encephalopathy/kernicterus.
Diagnostics (Basel). 2019;9:24.
4. Shapiro SM. Chronic bilirubin
encephalopathy: Diagnosis and outcome. Semin Fetal Neonatal
Med. 2010;15:157-63.
5. Wan A, Mat Daud S, Teh SH, et al.
Management of neonatal jaundice in primary care. Malaysian
Fam Physician. 2016;11:16-9.
6. Varughese, P. Kramer’s scale or
transcutaneous bilirubinometry: The ideal choice of a
paediatrician? Can we trust our eyes? International Journal
of Contemporary Pediatrics. 2019;6:1794-801.
7. Cheng NY, Lin YL, Fang MC. Noninvasive
transcutaneous bilirubin assessment of neonates with
hyperbilirubinemia using a photon diffusion theory-based
method. Biomed Opt Express. 2019;10:2969-84.
8. Tan KL, Dong F. Transcutaneous
bilirubinometry during and after phototherapy. Acta Paediatr.
2003;92:327-31.
9. Hulzebos CV, Vader-van Imhoff DE, Bos
AF, et al. Should transcutaneous bilirubin be measured in
preterm infants receiving phototherapy? The relationship
between trans-cutaneous and total serum bilirubin in preterm
infants with and without phototherapy. PLoS One. 2019;14:
e0218131
10. Taylor JA, Stout JW, de Greef L, et
al. Use of a smartphone app to assess neonatal jaundice.
Pediatrics. 2017;140: e20170312.
11. Doreswamy SM, Vasudev PH,
Thandaveshwara D. Stool color test can help to decide which
infants with neonatal hyperbilirubinaemia need phototherapy.
Acta Paediatr. 2018;108:371-72.
12. Webster J. An appraisal of the use of
the Kramer’s scale in predicting hyperbilirubinaemia in
healthy full-term infants. Birth Issues. 2006;14:83-89.
13. Olusanya BO, Ogunlesi TA, Kumar P, et
al. Management of late-preterm and term infants with
hyperbilirubinaemia in resource-constrained settings. BMC
Pediatr. 2015;15:39.
14. Slusher TM, Angyo IA, Bode-Thomas F,
et al. Transcu-taneous bilirubin measurements and serum
total bilirubin levels in indigenous African infants.
Pediatrics. 2004; 113:1636-41.
15. Gupta B, Chaudhary N, Bhatia B, et al. Non-invasive
trans-cutaneous bilirubin as a screening test to identify the
need for serum bilirubin assessment in healthy term neonates.
Journal of Universal College of Medical Sciences. 2014;1:17-21.
|
|
|
 |
|

