|
|
|
Indian Pediatr 2021;58: 928-931 |
 |
Prognostic Value of Amplitude-Integrated
Electroencephalography in Term Neonates With Encephalopathy
|
Giriraj Kumar Sharma, Chandra Kumar Natarajan, Vaanathi
Hementhakumar, Shanmuga Sundaram,
Shyam sundar Sharma
From Department of Neonatology, Kanchi Kamakoti CHILDS
Trust Hospital, Chennai, Tamil Nadu.
Correspondence to: Dr Chandra Kumar Natarajan, Head of
the Department, Department of Neonatology, Kanchi Kamakoti
CHILDS Trust Hospital, Nageshwara road, Nungambakkam,
Chennai, Tamil Nadu.
[email protected]
Received: October 22, 2020;
Initial review: December 15, 2020;
Accepted: April 10, 2021.
Published online: April 17, 2021, 2021;
PII:S097475591600311
|
Objective: To evaluate the prognostic
value of amplitude-integrated EEG in term neonates with
encephalopathy. Methods: In this prospective
observational study we enrolled 58 term neonates with
encephalopathy from March, 2019 to March, 2020. Level of
alertness was ascertained as per Volpe’s classification and
tone as per Amiel-Tison scale of tone assessment. Abnormal
aEEG was defined as background activity other than
continuous normal voltage, or immature or absent sleep-wake
cycle, or presence of electrical seizure. Primary outcome
was abnormal neurological examination at discharge and/or
death prior to discharge. Results: Out of 58
neonates, aEEG was abnormal for 50 (86.2%). There was a
statistically significant association between abnormal aEEG
findings and primary outcome (P=0.04). The aEEG score
cut-off of >2 had satisfactory sensitivity (88.8%) and
specificity (79.5%) to predict primary outcome.
Conclusion: Abnormal aEEG had good sensitivity but low
specificity to predict the primary outcome in term neonates
with encephalopathy.
Keywords: Hypoxic-ischemic encephalopathy,
Prognosis, Seizures, Sleep-wake cycle.
|
M
ulti-channel
electroencephalography
(EEG) is considered as the ‘gold standard’
for evaluating background activity and
detecting seizures, but continuous video-EEG monitoring and
its interpretation is not feasible in the majority of
neonatal intensive care units (NICUs), so
amplitude-integrated EEG (aEEG) is used for real time
monitoring of brain function and early detection of neonatal
seizures [3]. In aEEG, the raw EEG signals are filtered,
amplified and compressed for time to get a simplified aEEG
waveform by which we can monitor long term trends in
electro-cortical background activity [4].
Usefulness of aEEG in neonatal
encephalopathies other than hypoxic ischemic encephalopathy
has not been well studied and there is paucity of Indian
data regarding the utility of aEEG monitoring in neonates.
This study was planned to evaluate prognostic value of aEEG
in term neonates with encephalopathy.
METHODS
This prospective observational study was
conducted at a tertiary care NICU in India from March, 2019
to March, 2020 after institutional ethics committee
clearance and informed consent from parents of all
participants. Term neonates between 37 +0
to 41+6 weeks of
gestational age with encephalopathy were included. Babies
with major lethal congenital malformations, chromosomal
anomalies, neuronal migration disorders and myopathic
disorders were excluded.
Encephalopathy was defined as subnormal
alert state/altered neurological function which may be
associated with seizures [5]. Etiology of
encephalopathy was sub-grouped as hypoxic-ischemic
encephalopathy (HIE), infective causes, transient metabolic
causes, intracranial hemorrhage (ICH), dyselectrolytemia,
and inborn errors of metabolism (IEM). A detailed
neurological examination was performed, and level of
alertness was defined as per the Volpe classification, and
tone was assessed as per Amiel-Tison scale of tone
assessment [6,7].
The aEEG monitoring was done by CFM
Olympic Brainz Monitor (Natus Medical Inc.), with five
biparietal hydrogel electrodes. Electrode impedance was
main-tained below 10 ohms during monitoring. The aEEG was
monitored for at least 24 hours for all enrolled neonates. A
single investigator, trained for interpretation of aEEG
findings in 10 aEEG recordings by a pediatric neurologist,
noted the aEEG findings and scored as per the scoring used
by Zhang, et al. [8].
Presence of any one of the following was
defined as abnormal aEEG: any background activity other than
continuous normal voltage, immature or absent sleep-wake
cycle (SWC), and presence of any electrical seizure. Primary
outcome was defined as subnormal level of alertness as per
Volpe classification or any tone abnormality as per
Amiel-Tison scale, at the time of discharge and/or death
before discharge. Secondary outcomes were abnormal
background activity, abnormal SWC, and electrical seizures,
as defined above.
Statistical analysis: SPSS Software
version 16 was used for data analysis. To determine the
association between categorical variables, Chi square test
was used as test of significance. P<0.05 was
considered statistically significant. Diagnostic efficacy of
aEEG background activity, aEEG sleep-wake cycle and aEEG
seizures in predicting outcome at discharge was assessed by
calculating specificity, sensitivity, positive predictive
value (PPV), negative predictive value (NPV), positive
likelihood ratio and negative likelihood ratio. Diagnostic
efficacy of cumulative aEEG score in predicting outcome was
assessed by ROC curve, suitable cut-off values were selected
and AUC was calculated.
RESULTS
Of all the term neonates with
encephalopathy, 58 were finally enrolled in the study and
followed-up till discharge or death (Fig. 1). The
baseline characteristics are given in Table I. Out of
these, 50 (86.2%) survived. aEEG findings were abnormal for
50 (86.2%) of enrolled neonates. Out of these, 31 (62%) had
normal outcome and 19 (38%) had abnormal outcome at
discharge, or died prior to discharge. There was a
statistically significant association between abnormal aEEG
findings and primary outcome. Abnormal aEEG had 100%
sensitivity, 20.5% specificity, 38% PPV, 100% NPV, positive
likelihood ratio 1.26 and negative likelihood ratio 0, to
predict primary outcome (Web Table I).
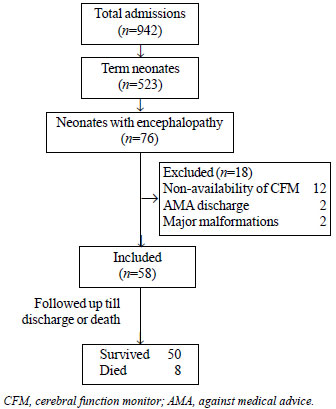 |
|
Fig. 1 Study flow diagram.
|
Table I Baseline Characteristics of Study Population (N=58)
|
Gestational age, wka |
38.5 (1.14) |
| Birthweight, g |
2889 (360) |
| Male gender |
35 (60.3) |
| Postnatal age |
|
| 1-3 d |
36 (62) |
| 4-7 d |
8 (14) |
| 8-14 d |
5 (8.6) |
| 15-28 d |
9 (15.5) |
| Mode of delivery |
|
| Vaginal delivery |
24 (41) |
| Assisted vaginal delivery |
6 (10) |
| Caesarean section |
28 (48) |
| Resuscitation at birth |
30 (52) |
| Antenatal risk factors |
|
| Fetal distress |
14 (24) |
| Pregnancy induced hypertension |
6 (10) |
| Oligohydramnios |
7 (12) |
| Polyhydramnios |
6 (10) |
| Premature rupture of membranes |
4 (7) |
| Etiology of encephalopathy |
|
| Hypoxic ischemic encephalopathy |
36 (62) |
| Infective |
12 (21) |
| Transient metabolic |
4 (7) |
|
Intracranial hemorrhage |
2 (3) |
| Dyselectrolytemia |
2 (3) |
| Inborn error of metabolism |
2 (3) |
| Variables are
expressed as n (%) except amean (SD). |
Out of 58 neonates, 38 (65.5%) had
abnormal back-ground activity. There was a statistically
significant association between background activity and
primary outcome (P=0.001). Out of 58 neonate, 19
(32.7%) had mature sleep-wake cycle and 39 (67.2%) neonates
had immature or absent sleep-wake cycle, and there was a
statistically significant association between sleep-wake
cycle and primary outcome (P=0.001). A total of 43
(74.1%) neonates had electrical seizures, and aEEG seizures
were significantly associated with primary outcome (P=0.002).
A cumulative aEEG score of 0-2 was seen
in 32 (55%) neonates, and 26 (45%) had score >2. Out of 32
neonates with score of 0-2, 31 (97%) had normal outcome, ROC
curve was plotted and cut-off value >2 was selected for high
sensitivity and low false positive rate. There was a
statistically significant association between cumulative
aEEG score >2 and primary outcome (P=0.001) (Fig.
2).
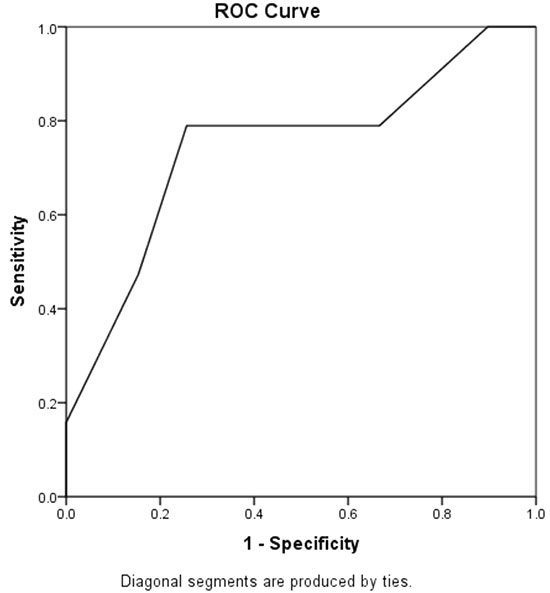 |
|
Fig. 2 Receiver operator
characteristic (ROC) curve of cumulative aEEG
scoring to predict abnormal neurological outcome
(AUC=0.746).
|
DISCUSSION
In this prospective observational study
we assessed the characteristics of aEEG in 58 term neonates
with encephalopathy and found that background activity,
sleep-wake cycle and electrical seizures were significantly
associated with primary outcome. In this study we also
calculated cumulative aEEG scoring and we found that
cumulative aEEG score of >2 was significantly associated
with primary outcome. These results were largely consistent
with previous studies.
In the present study we found that
abnormal aEEG was significantly associated with abnormal
outcome and it has high sensitivity, but low specificity to
predict primary outcome. A meta-analysis by Chandrasekaran,
et al. [9] showed similar results with pooled sensitivity of
87% and specificity of 36%. Similar to our findings, Van der
Heide, et al. [10] noted significant association between
aEEG background activity and neurologic outcome in neonates.
Sewell, et al. [11] showed results opposite to our study
with low sensitivity and high specificity. This may be due
to inclusion of all grades of encephalopathy with various
etiologies in our study, while they only included neonates
with HIE. We found that aEEG cyclicity was significantly
associated with primary outcome. Rhie, et al. [12]
con-cluded in their study that delayed appearance of SWC was
significantly associated with unfavorable neuroimaging in
neonates with HIE, as was also seen in our study for all
causes of encephalopathy.
Variane, et al. [13] showed that presence
of recurrent aEEG seizures were associated with MRI brain
abnormality and death, similar to this significant
association with outcome was found in our study. Similar to
this study, Luo, et al. [14] showed that aEEG scoring system
has a higher specificity but low sensitivity as compared to
individual components for abnormal outcomes.
Strengths of our study include enrolment
of subjects with various causes of encephalopathy, albeit
majority were HIE, and detailed study of the individual
components of aEEG tracing and formulation of cumulative
scoring cut-offs to predict short term neurological outcome.
Limitations of our study include a
relatively small sample size, and inability to study long
term neurological outcomes. Neonates admitted in the late
stage of encephalopathy could have had different findings in
aEEG if we had recorded aEEG at the onset of encephalopathy.
Effect of ongoing drugs, and thera-peutic hypothermia were
not taken into account, and the effects of postnatal age on
aEEG findings were not studied.
To conclude, aEEG parameters such as
abnormal background activity, absent sleep-wake cycling and
presence of electrical seizures, either alone or in
combination are associated with primary outcome of subnormal
level of alertness or tone abnormality at discharge in term
neonates with encephalopathy.
Note: Additional material related to this
study is available with the online version at
www.indianpediatrics.net
Ethical clearance: Institutional
ethics committee, Kanchi Kamakoti CHILDS Trust Hospital
Chennai; No. IEC-DNB/26/February2019, dated March 11, 2019.
Contributors: SGK: conceptualized the
study, collected data, wrote the first draft of manuscript;
NCK: study design, analysis, corrected manuscript and
approved for final submission; HV: critical review of
proposal, expert advice on data analysis and interpretation;
SS: protocol development, supervising enrolment and outcome
assessment; SSS: participated in planning of project and
writing manuscript. All authors approved the final version
of manuscript, and are accountable for all aspects related
to the study.
Funding: None; Competing interest:
None stated.
|
WHAT THIS STUDY ADDS?
• Abnormal aEEG has high
sensitivity but low specificity to predict primary
outcome of subnormal level of alertness or tone
abnormality at discharge, or death before discharge
in term neonates with encephalopathy.
|
REFERENCES
1. Glass HC, Kan J, Bonifacio SL, et
al. Neonatal seizures: Treatment practices among term
and preterm infants. Pediatric Neurol. 2012;46:111-5.
2. Hellström-Westas L, De Vries LS,
Rosén I. An Atlas of Amplitude-integrated EEGs in the
Newborn. CRC Press; 2008.
3. Shah DK, Boylan GB, Rennie JM.
Monitoring of seizures in the newborn. Arch Dis Child
Fetal Neonatal Ed. 2012;97:F65-9.
4. Hellström-Westas L, Rosén I, De
Vries LS, et al. Amplitude-integrated EEG classification
and interpretation in preterm and term infants. Neo Rev.
2006;7:e76-87.
5. D’Alton Mary E, Hankins GD,
Berkowitz RL, et al, Neonatal encephalopathy and
neurologic outcome. Pediarics. 2014;133;e1482.
6. Volpe JJ. Neurological
examination: normal and abnormal features. In:
Volpe’s Neurology of the Newborn, sixth edition,
Elsevier. 2018;191-221.
7. Gosselin J, Gahagan S, Amiel Tison
C. The Amiel Tison neurological assessment at term:
Conceptual and methodological continuity in the course
of follow up. Mental Retardation Dev Disab Res Rev.
2005;11:34-51.
8. Zhang D, Ding H, Liu L, et al. The
prognostic value of amplitude-integrated EEG in
full-term neonates with seizures. PLoS One.
2013;8:e78960.
9. Chandrasekaran M, Chaban B,
Montaldo P, et al. Predictive value of
amplitude-integrated EEG (aEEG) after rescue hypothermic
neuroprotection for hypoxic ischemic encephalopathy: A
meta-analysis. J Perinatol. 2017;37: 684.
10. Van der Heide MJ, Roze E, van der
Veere CN, et al. Long-term neurological outcome of
term-born children treated with two or more
anti-epileptic drugs during the neonatal period. Early
Human Dev. 2012;88:33-8.
11. Sewell E K, Vezina Gilbert, Chang
Taeun, et al. Evolution of amplitude-integrated
electroencephalogram as a predictor of outcome in term
encephalopathic neonates receiving therapeutic
hypothermia. Amer J Perinatol. 2018;35:277-85.
12. Rhie S, Chae KY, Jo HS, et al.
Sleep-wake cycle on amplitude-integrated EEG and
neuroimage outcomes in newborns. Italian J Pediatr.
2016;42:85.
13. Variane GF, Magalhaes M,
Gasperine R, et al. Early amplitude-integrated
electroencephalography for moni-toring neonates at high
risk for brain injury. Jornal de Pediatria.
2017;93:460-6.
14. Luo F, Chen Z, Lin H, et al. Evaluation of cerebral
function in high risk term infants by using a scoring system
based on aEEG. Translat Pediatr. 2014;3:278.
|
|
|
 |
|

