|
|
|
Indian Pediatr 2021;58: 922- 927 |
 |
Preterm White Matter Injury: A Prospective
Cohort Study
|
|
Mohsin Raj Mantoo, 1 Ashok
K Deorari,1 Manisha Jana,2
Ramesh Agarwal,1 M Jeeva
Sankar,1
Anu Thukral1
From the Departments of 1Pediatrics and 2Radiodiagnosis, All India
Institute of Medical Sciences, New Delhi.
Correspondence to: Dr Ashok K Deorari, Professor and Head, Department
of Pediatrics, All India Institute of Medical Sciences, New Delhi 110
029.
Email: [email protected]
Received: January 05, 2021;
Initial review: February 09, 2021;
Accepted: July 19, 2021.
Published online: July 23, 2021;
PII: S097475591600356
|
|
Objective: To determine the
incidence and risk factors of preterm white matter injury [WMI;
periventricular-intraventricular hemorrhage (PIVH) and/or
periventricular leukomalacia (PVL)].
Design: Prospective cohort study.
Setting: Level-3 neonatal
intensive care unit.
Patients: Inborn preterm neonates
(n=140) delivered at <32 weeks gestation or birthweight <1500 g.
Methods: Serial cranial
ultrasounds were performed at postnatal ages of 3 days (±12 hour), 7
(±1) days, 21 (±3) days and 40 (±1) weeks postmenstrual age (PMA). PIVH
and PVL were graded as per Volpe and De-Vries criteria, respectively.
Univariate followed by multivariate analysis was done to evaluate risk
factors for PIVH and PVL.
Outcome measures: The primary
outcome was the incidence of preterm WMI. The secondary outcomes were
evaluation of risk factors and natural course of WMI.
Results: The mean (range)
gestation and birth weight of enrolled neonates were 29.7 (24-36) weeks
and 1143 (440-1887) g, respectively. PIVH occurred in 25 (17.8%)
neonates. PVL occurred in 34 (24.3%) neonates. None of them were grade
III or IV PVL. Preterm WMI (any grade PIVH and/or PVL) occurred in 52
(37.1%) neonates. Severe PIVH (grade III) and cystic PVL occurred in 7
(5%) and 5 (3.6%) neonates, respectively. On multivariate analysis, none
of the presumed risk factors were associated with PIVH. However,
hemodynamically significant patent ductus arteriosus, and apnea of
prematurity were significantly associated with increased risk of PVL.
Conclusions: Significant WMI
occurred only in one-third of the cohort, which is comparable to that
described in literature from the developed countries.
Keywords: Outcome, Periventricular-
intraventricular hemorrhage, Periventricular leukomalacia, Risk factors.
|
|
Survival of very low birthweight (VLBW) neonates has
improved over the last decade; survival rates being more than 90% in
most centers [1]. This is largely due to improved obstetric and neonatal
care practices, in particular, use of antenatal steroids and gentler
non-invasive modes of ventilation [2]. Preterm white matter injury (WMI)
and its associated neurological sequelae are of important concern to the
neonatologist.
Periventricular-intraventricular hemorrhage (PIVH)
and periventricular leukomalacia (PVL) are the two major forms of
preterm WMI. PIVH originates in a highly active zone of cell
proliferation in the preterm brain called as subependymal zone or
germinal matrix, and later spreads into the ventricular cavity. PVL is
characterized by foci of necrosis in periventricular white matter (focal
component) and more diffuse glial response in the form of reactive
gliosis and microglial activation in the surrounding white matter
(diffuse component). Cranial ultrasound (CUS) is the screening procedure
of choice for preterm WMI, especially PIVH [3].
Initial studies done in the 1980s showed a high
incidence of PIVH (up to 40-50%) [4-6]. This has decreased to about
20-25% in developed countries in the last two decades [7,8]. In a study
from our own center in 2004, the incidence of preterm WMI was about 32%
[9]. No recent data is
available from low-middle income countries like India. Hence, we planned
this prospective cohort study to determine the incidence of WMI and its
associated risk factors in VLBW neonates. The primary objective of this
study was to determine the incidence of PIVH and PVL (i.e., preterm WMI)
in neonates born at less than 32 weeks gestation or birth weight <1500
g. The secondary objectives were to evaluate the risk factors and
natural history of PIVH and PVL till discharge from Neonatal intensive
care unit (NICU) or 40 weeks postmenstrual age (PMA) using serial CUS.
METHODS
This prospective cohort study was conducted at a
level-3 NICU, All India Institute of Medical Sciences, New Delhi from
March, 2018 to June, 2019. Inborn preterm neonates born at <32 weeks
gestation or birth weight <1500g were enrolled. Neonates with major CNS
malformations (antenatally diagnosed or diagnosed at birth) or dying
before the first cranial ultrasound were excluded. The neonates were
followed until 40 weeks PMA or discharge from NICU, whichever was later
(Fig. 1).
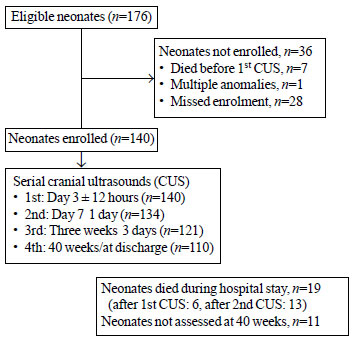 |
|
Fig. 1 Study flow chart.
|
The study was approved by the institutional ethics
committee. Before enrolment an informed consent was obtained from
parents.
Protocol for cranial ultrasound: Serial cranial
ultrasounds (CUS) were performed on: Day 3±12 hours; Day 7±1 day;
three weeks ± 3 days and 40 weeks/at discharge. If any CUS
other than the last one was abnormal, clinical team decided frequency of
next ultrasound. All initial CUS (within the first week) were performed
bedside using Philips CX-50 (Philips). Subsequent CUS were done bedside
wherever feasible (neonate in level 3 unit) or in the Radiology
department (for neonates admitted in step down unit after stabilization)
using Supersonic imagine (Aixplorer) or Antaris (Siemens).
Using a small footprint curvilinear probe with 2-5
MHz or higher frequency, CUS was performed using anterior fontanelle as
an acoustic window. Standard views were obtained in the coronal and
sagittal planes. Ventricular index (VI) was measured in coronal plane as
the distance between falx and lateral end of anterior horn of lateral
ventricle [10]. Thalamo-occiptal distance (TOD) was measured in oblique
parasagittal view as distance between the outermost point of thalamus at
its junction with choroid plexus and outermost extent of occipital horn
[10].
An experienced pediatric radiologist performed the
cranial ultrasound (CUS) in first 40 cases. Subsequently, CUS were done
by the first author with a simultaneous review of all images done by the
pediatric radiologist. There was < 10% discrepancy in the reported
findings and in all such cases, report of the radiologist was considered
final. PIVH was graded as per Volpe classification and PVL as per De
Vries criteria [11,12]. Transient periventricular flare was defined as
periventricular echogenicity not lasting for more than 7 days. The
reference values of VI and TOD, were from the study by Brouwer, et al.
[10].
Gestational age was assessed from the last menstrual
period or first trimester ultrasound scan. If both were not available or
a discrepancy of 1 week or greater was noted between the two, expanded
New Ballard score was used [13]. Steroid coverage was considered
‘complete’, if the mother received 4 doses of dexamethasone 12 hours
apart or 2 doses of betamethasone 24 hours apart, with last dose given
at least 24 hours before delivery. Small for gestational age (SGA) was
defined as birth weight less than 10th percentile on Lubchenco charts
14]. Birth asphyxia was defined as 5 minute Apgar score
£5. Respiratory
distress syndrome (RDS), sepsis, hemodyna-mically significant PDA
(hs-PDA), shock, preterm premature rupture of membranes (PPROM) and
prolonged rupture of membranes (PROM) were defined as per standard
definitions [15]. Arterial blood gases (ABG) done by the NICU team for
clinical monitoring were checked for PaCO2 values in first 72 hours of
birth. Hypocapnia was defined as PaCO2 <35 mmHg and hypercarbia as PaCO2 >45 mmHg. Hypotension
was defined as BP < 5th centile [16].
The antenatal details like demographic data,
obstetric history and current pregnancy details including antenatal
steroid status, gestational age, cause of preterm delivery, duration of
rupture of membranes, etc. were collected by principal investigator from
mother’s hospital records after the baby was enrolled. The details of
delivery room resuscitation and NICU course like sepsis, hypotension,
shock, hypocapnia, hypercarbia and acidosis in first 72 hours, were
recorded in a predesigned proforma.
Based on the available literature, we assumed the
prevalence of preterm WMI as 20%. With 95% confidence limit and an
absolute precision of 7%, the sample size was calculated to be 126.
Anticipating a loss to follow up at 40 weeks PMA, we decided to enroll
140 neonates.
Statistical analysis: A database was created in
Microsoft Access 2016 (Microsoft) for recording the variables. Data
analysis was done using STATA 15.1 version (Stata Corp). The incidence
of PIVH and PVL along with 95% confidence intervals (95% CI) were
calculated. For risk factors, we did univariate analysis followed by
multivariate logistic regression with PIVH or PVL as dependent variable
and identified risk factors as independent variables. Data analysis was
done separately for PIVH and PVL.
RESULTS
During the study period, 176 eligible neonates were
born, of which 140 were enrolled (Fig. 1). The mean
(range) gestation and birthweight of enrolled neonates were 29.7 (24-36)
week and 1143 (440-1887) g, respectively. The baseline characteristics
of enrolled neonates are summarized in Table I.
Table I Baseline Characteristics and Hospital Course of Preterm Neonates Enrolled in the Study (N=140)
| Characteristics |
N (%) |
| Maternal characteristics |
|
| Hypertension |
30 (21.4) |
| Diabetes |
30 (21.4) |
| PPROM |
52 (37.1) |
| Antenatal steroids |
130 (92.8) |
| Complete course |
89 (63.6) |
| Vaginal delivery |
24 (17.1) |
| Delivery room details |
|
|
5-min Apgar score £5 |
26 (18.6) |
| Delivery room CPAP |
101 (72.1) |
| Delivery room intubation |
36 (25.7) |
| Chest compressions |
3 (2.14) |
| Neonatal characteristics |
|
| Male gender |
79 (56.4) |
| Small for date |
50 (35.7) |
|
Gestational agea |
29.7 (24-36) |
| < 28 wks |
26 (18.6) |
| 28-31 wks |
79 (56.4) |
| ³ 32 wks |
35 (25) |
| Birthweight (g) a |
1143 (440-1887) |
| <500 |
2 (1.4) |
| 500-749 |
15 (10.7) |
| 750-999 |
36 (25.7) |
| 1000-1499 |
70 (50) |
| ³1500 |
17 (12.1) |
| RDS requiring surfactant |
40 (28.6) |
| CPAP in NICU |
121 (86.4) |
| IMV in NICU |
49 (35) |
|
Hypocapnia within 72 hb |
9 (6.4) |
|
Hypercarbia within 72 hb |
15(10.7) |
|
Acidosis within 72 hb |
19(13.6) |
|
Shock within 72 hb |
8 (5.7) |
| Shock at any time |
22 (15.7) |
| hsPDA |
28 (20) |
| Sepsis |
51 (36.4) |
| Meningitis |
6 (4.3) |
|
Necrotizing enterocolitis |
4 (2.9) |
|
Data presented as no. (%) or amean (range). bHours of life.
PPROM: Preterm premature rupture of membranes; CPAP: Continuous
positive airway pressure; RDS: Respiratory distress syndrome;
IMV: Invasive mechanical ventilation; hsPDA: Hemodynamically
significant patent ductus arteriosus; NICU: neonatal intensive
care unit. |
PIVH occurred in 25 (17.8%; 95% CI 12.3–25.2%)
neonates-grade I, II, III, and IV in 17 (12.1%), 1 (0.7%), 6 (4.3%), and
1 (0.7%) neonates, respectively. Severe PIVH (grade III) occurred in 7
(5%) neonates. PIVH was bilateral in 15 neonates. PVL occurred in 34
(24.3%; 95% CI 17.8–32.2%) neonates-grade I and II in 29 (20.7%) and 5
(3.6%) neonates, respectively. Transient periventricular flares occurred
in 7 neonates. We did not detect any grade III or IV PVL. Cystic PVL
occurred in 5 (3.6%) neonates. PVL was bilateral in all our neonates.
Preterm white matter injury (any grade PIVH and/or PVL) occurred in 52
(37.1%; 95% CI 29.5-45.5%) neonates (Table II).
Table II Profile of Preterm White Matter Injury Among the Study Cohort (N=140)
| Characteristic |
No. (%) [95% CI] |
|
Peri-intraventricular hemorrhage |
|
| Any grade |
25 (17.8) [12.3-25.2] |
| Worst grade |
|
| I |
17 (12.1) |
| II |
1 (0.7) |
| III |
6 (4.3) |
| IV (PVHI) |
1 (0.7) |
|
Periventricular leukomalacia (PVL)
|
|
| Any grade |
34 (24.3) [17.8-32.2] |
| Worst grade |
|
| I |
29 (20.7) |
| II |
5 (3.6) |
| IIIIV |
00 |
| Cystic PVL |
5 (3.6) |
|
Preterm white matter injurya
|
52 (37.1) [29.4-45.5] |
PIVH was detected in first three days of life in 22
(88%) neonates and in all cases by day 7. Progressive ventricular
dilation occurred in one-third of patients with grade III PIVH.
Periventricular white matter echogeni-cities appeared in 33 (97%) cases
within the first 7 days of life. Periventricular cysts were detected in
5 cases of cystic PVL by 3 weeks of age. Additional three neonates had
ventricular dilation in CUS done at term gestation with no abnormality
detected in previous scans, indicating likely periventricular white
matter loss.
On univariate analysis, female gender, vaginal
delivery, hypercarbia, acidosis and shock in first 72 hours of life were
associated with increased risk of PIVH. However, on multivariate
analysis none of these factors were significant. Similarly,
delivery room endotracheal intubation, hypocapnia in first 72 hours of
life, apnea, anemia requiring transfusion, hsPDA, sepsis, meningitis and
BPD were associated with PVL on univariate analysis. However, on
multivariate analysis, only hsPDA (OR 3.09; 95% CI 1.02–9.39; P=0.04)
and apnea (OR 2.81; 95% CI 1.04-7.56; P=0.04) were found to be
significantly associated with increased risk of PVL (Table III).
Table III Risk Factors of Periventricular Leukomalacia (PVL) Among the Study Cohort (N=140)
|
PVL (n=34) |
No PVL (n=106) |
Adjusted OR(95% CI) |
P value |
| Male gender |
19 (55.9) |
60 (56.6) |
1.07 (0.47-2.48) |
0.86 |
| Small for gestational age |
16 (47) |
34 (32.1) |
2.48 (0.94-6.54) |
0.06 |
| Prolonged rupture of membranes |
4 (1.2) |
19 (17.9) |
0.32 (0.07-1.47) |
0.15 |
| Preterm premature rupture of membranes |
14 (41.2) |
38 (35.8) |
2.69 (0.79-9.14) |
0.11 |
| Antenatal steroids |
20 (58.8) |
69 (65.1) |
1.31 (0.50-3.39) |
0.58 |
| Vaginal delivery |
8 (23.5) |
16 (15.1) |
0.72 (0.17-3.05) |
0.65 |
|
5-min Apgar score <5 |
8 (23.5) |
18 (6.9) |
1.57 (0.50-4.94) |
0.43 |
| Respiratory distress syndrome |
10 (29.4) |
30 (28.3) |
0.47 (0.13-1.47) |
0.18 |
| Apnea |
23 (67.6) |
39 (36.8) |
2.81 (1.04-7.56) |
0.04 |
|
Shock requiring inotropes |
8 (23.5) |
14 (13.2) |
1.01 (0.29-3.57) |
0.73 |
|
Hemodynamically significant patent ductus arteriosus
|
12 (35.3) |
16 (15.1) |
3.09 (1.02-9.39) |
0.04 |
| Meningitis |
4 (11.8) |
2 (1.9) |
2.05 (0.24-17.4) |
0.50 |
DISCUSSION
In this cohort of 140 neonates, PIVH occurred
in 25 (17.8%) neonates. Severe PIVH occurred in 7 (5%) neonates. PVL
occurred in 34 (24.3%) and cystic PVL occurred in 5 (3.6%) neonates.
Preterm WMI (any grade PIVH and/or PVL) occurred in 52 (37.1%) neonates.
However, most neonates had low-grade lesions (grade I PIVH/PVL). Severe
PIVH and cystic PVL occurred in less than 5% of neonates which is
comparable to what is described in literature from developed countries.
Comparing our findings to a previous study done from
our own center 17 years back by Maria et.al [9], the incidence of any
grade PVL and cystic PVL decreased from 36.2% and 12.4% to 24.3% and
3.6%, respectively. The incidence of cystic PVL in our cohort is similar
to that reported in data from developed world [17-18]. Studies from
1980s reported the incidence of PIVH in premature neonates up to 40-50%
[4-7]. The incidence decreased to 20-25% in studies done in late 1980s
and 1990s [8,19]. The incidence of PIVH has remained almost the same in
the last decade i.e., 20-25% in VLBW and up to 40% in neonates born at
d" 28 weeks gestation [17,20]. However, there has been an increase in
the survival of ELBW neonates who are at even higher risk of PIVH, which
may mask a true decline in incidence of PIVH.
Multiple studies have implicated the role of pressure
passive cerebral circulation of preterm neonates in causation of PIVH;
[18] the classic setting being severe RDS requiring mechanical
ventilation [21-22]. Hypercarbia also increases the risk of PIVH [23].
However, in our study, none of these risk factors were associated with
PIVH. In our cohort, vaginal delivery was not associated with increased
risk of PIVH. Earlier studies had shown that VLBW neonates born
vaginally were at higher risk of PIVH [24]. However, more recent studies
have contrary results [25]. In a study on periventricular hemorrhagic
infarction (PVHI) by Bassan, et al. [26], fetal distress, need for
emergency cesarean section, low Apgar scores, and need for respiratory
resuscitation were strongly associated with PVHI. Another interesting
study in which 95 VLBW infants underwent amplitude- integrated EEG
monitoring for first 72 hours of life found high incidence (48%) of
seizures which increased the risk for IVH and white matter injury [27].
Our study failed to show any association of delivery room resuscitation
or birth asphyxia with PIVH. In this study, hsPDA and apnea were
associated with increased risk of PVL. We, however, did not find any
significant association of PVL with low APGAR scores, RDS, hypocapnia,
acidosis, PPROM or meningitis, contrary to what is described in
literature [28-31].
In our study, PIVH was detected in first three days
of life in 22 (88%) cases and in all 25 (100%) cases by day 7. Most
cases of PIVH were clinically silent. Approximately 90% of cases of IVH
occur within the first 72 hours of life, with 50% occurring in first 6
hours [5,32,33]. The lesion progresses in about 10-20% cases over 3-5
days [34].
We had 3 neonates with apparently normal first 3 CUS
scans, but showed ventriculomegaly in CUS done at term equivalent age.
Data suggests that presence of persistent echo-densities for >7 days is
significant and may actually represent non-cystic PVL [35,36]. In a
study by Inder et.al, on 96 VLBW neonates, 10 neonates who had either
normal CUS or transient echodensity had subsequent evidence of WMI on
MRI at term. Further, 22 neonates with overtly abnormal CUS as
persistent echo-density had normal MRI at term gestation. Therefore, the
sensitivity and specificity of transient and persistent echodensity on
CUS for predicting abnormal MRI findings at term may not be good [35].
Therefore, while periventricular cysts are sensitive and specific for
abnormal MRI correlates and poor neurodevelopmental outcomes, transient
and persistent echodensities/flares are variably predictive of WMI on
MRI at term gestation. We decided to follow PVL using CUS only because
of the ease of doing bedside CUS and the risks involved in doing MRI
under anesthesia.
The strengths of this study are its prospective
cohort design and meticulous follow up of VLBW neonates till term
gestation. About 80% of enrolled neonates underwent at least four CUS.
All CUS images were reviewed by an expert pediatric radiologist. Data
analysis was done separately for PIVH and PVL.
Our study has some limitations too. The study was not
powered to evaluate the risk factors of WMI. We chose a relatively
larger margin of precision (7%) primarily due to feasibility
considerations. In addition, MRI brain would have been a better modality
for characterization of PVL. However, literature does suggest acceptable
agreement between serial CUS and MRI done at 40 weeks’ gestation [37].
We also could not assess the impact of the CUS abnormalities on
subsequent neuromotor development.
In conclusion, most VLBW neonates in our cohort had
low-grades of preterm WMI (grade I PIVH and PVL). The incidence of
severe PIVH and cystic PVL in our setting is low and is comparable to
data from developed countries. We also noticed a decrease in the
incidence of preterm WMI over the last 15 years in our setting.
Note: Additional material related to this
study is available with the online version at
www.indianpediatrics.net
Ethics clearance: Institutional ethics committee,
All India Institute of Medical Sciences, New Delhi; No. IECPG-457, dated
November 29, 2017.
Contributors: MRM: principal investigator;
reviewed literature; prepared the initial protocol and this manuscript;
collected data; performed cranial ultrasounds of enrolled neonates with
images reviewed by MJ; AKD: framed the idea and rationale of this study;
reviewed the protocol; supervised this study throughout its course;
critical revision and finalization of this manuscript; MJ: Framed the
cranial ultrasound (CUS) protocol; performed CUS in first 40 cases and
reviewed all CUS images; RA,JS,AS: helped in preparation of initial
protocol and this manuscript; supervised the study and critical revision
of this manuscript.
Funding: None; Competing interests:
None stated.
|
WHAT IS ALREADY KNOWN?
•
The
incidence of preterm white matter injury (any grade) and severe
peri-intraventricular hemorrhage (PIVH) /cystic periventricular
leucomalacia (PVL) is 20-25% and below 5%, respectively in the
developed countries.
•
Incidence of any grade PVL and cystic PVL in India 15 years
ago was 36.2% and 12.4%, respectively.
WHAT THIS STUDY ADDS?
•
Severe PIVH and cystic PVL occurred in less than 5% of
neonates.
|
REFERENCES
1. Survival and Morbidity of Preterm Children
Born at 22 Through 34 Weeks’ Gestation in France in 2011: Results of
the EPIPAGE-2 Cohort Study. JAMA Pediatr. [Internet]. 2019. Accessed
August 11, 2019. Available from:
https://jamanetwork.com/journals/jamapediatrics/fullarticle/2091623
2. Stoll BJ, Hansen NI, Bell EF, et al. Trends in
care practices, morbidity, and mortality of extremely preterm
neonates, 1993-2012. JAMA. 2015;314:1039-51.
3. van Wezel-Meijler G, Steggerda SJ, Leijser LM.
Cranial ultrasonography in neonates: role and limitations. Semin
Perinatol. 2010;34:28-38.
4. Hawgood S, Spong J, Yu VYH. Intraventricular
hemorr-hage: Incidence and outcome in a population of
very-low-birth-weight infants. Am J Dis Child. 1984;138:136-9.
5. Partridge JC, Babcock DS, Steichen JJ, et al.
Optimal timing for diagnostic cranial ultrasound in low-birth-weight
infants: Detection of intracranial hemorrhage and ventricular
dilation. J Pediatr. 1983;102:281-7.
6. Burstein J, Papile L, Burstein R.
Intraventricular hemorr-hage and hydrocephalus in premature
newborns: A pros-pective study with CT. Am J Roentgenol. 1979;132:
631-5.
7. McMenamin JB, Shackelford GD, Volpe JJ.
Outcome of neonatal intraventricular hemorrhage with periventricular
echodense lesions. Ann Neurol. 1984;15:285-90.
8. Marba STM, Caldas JPS, Vinagre LEF, et al.
Incidence of periventricular/intraventricular hemorrhage in very low
birthweight infants: A 15-year cohort study. J Pediatr (Rio J).
2011;87:505-11.
9. Maria A, Gupta A, Sreenivas V, et al.
Incidence of peri-ventricular leucomalacia among a cohort of very
low birth weight neonates (<1500 g). Indian Pediatr. 2006; 43:7.
10. Brouwer MJ, de Vries LS, Groenendaal F, et
al. New reference values for the neonatal cerebral ventricles.
Radiology. 2012;262:22433.
11. Inder TE, Perlman JM, Volpe JJ. Chapter 24 -
Preterm intraventricular hemorrhage/posthemorrhagic hydro-cephalus.
In: Volpe JJ, Inder TE, Darras BT, et al., editors. Volpe’s
Neurology of the Newborn (Sixth Edition). Elsevier; 2018. p.
637-698.e21.
12. de Vries LS, Eken P, Dubowitz LMS. The
spectrum of leukomalacia using cranial ultrasound. Behav Brain Res.
1992;49:1-6.
13. Ballard JL, Khoury JC, Wedig K, et al. New
Ballard Score, expanded to include extremely premature infants. J
Pediatr. 1991;119:417-23.
14. Lubchenco LO, Hansman C, Boyd E. Intrauterine
growth in length and head circumference as estimated from live
births at gestational ages from 26 to 42 weeks. Pediatrics.
1966;37:403-8.
15. Agarwal R, Deorari A, Paul V, et al. AIIMS
protocols in Neonatology. 2nd ed. Vol. 1. Noble; 2019. p.
733.
16. Zubrow AB, Hulman S, Kushner H, et al.
Determinants of blood pressure in infants admitted to neonatal
intensive care units: A prospective multicenter study. Philadelphia
Neonatal Blood Pressure Study Group. J Perinatol Off J Calif Perinat
Assoc. 1995;15:470-9.
17. Waitz M, Nusser S, Schmid MB, et al. Risk
factors associated with intraventricular hemorrhage in preterm
infants with ³28
Weeks gestational age. Klin Padiatr. 2016;228:245-50.
18. Soul JS, Hammer PE, Tsuji M, et al.
Fluctuating pressure-passivity is common in the cerebral circulation
of sick premature infants. Pediatr Res. 2007;61:467-73.
19. Paneth N, Pinto-martin J, Gardiner J, et al.
Incidence and timing of germinal matrix/intraventricular hemorrhage
in low birth weight infants. Am J Epidemiol. 1993;137:1167-76.
20. Wei JC, Catalano R, Profit J, et al. Impact
of antenatal steroids on intraventricular hemorrhage in
very-low-birth weight infants. J Perinatol. 2016;36:352-6.
21. Perlman JM, McMenamin JB, Volpe JJ.
Fluctuating cerebral blood-flow velocity in respiratory-distress
syndrome. Relation to the development of intraventricular
hemorrhage. N Engl J Med. 1983;309:204-9.
22. Van Bel F, Van de Bor M, Stijnen T, et al.
Aetiological rôle of cerebral blood-flow alterations in development
and extension of peri-intraventricular haemorrhage. Dev Med Child
Neurol. 1987;29:601-14.
23. Kaiser JR, Gauss CH, Pont MM, et al.
Hypercapnia during the first 3 days of life is associated with
severe intraventricular hemorrhage in very low birth weight infants.
J Perinatol. 2006;26:279-85.
24. Shankaran S, Bauer CR, Bain R, et al.
Prenatal and perinatal risk and protective factors for neonatal
intracranial hemorrhage. National Institute of Child Health and
Human Development Neonatal Research Network. Arch Pediatr Adolesc
Med. 1996;150:491-7.
25. Riskin A, Riskin-Mashiah S, Bader D, et al.
Delivery mode and severe intraventricular hemorrhage in single, very
low birth weight, vertex infants. Obstet Gynecol. 2008;112:21-8.
26. Bassan H, Feldman HA, Limperopoulos C, et al.
Periventricular hemorrhagic infarction: Risk factors and neonatal
outcome. Pediatr Neurol. 2006;35:85-92.
27. Vesoulis ZA, Inder TE, Woodward LJ, et al.
Early electrographic seizures, brain injury, and neurodevelop-mental
risk in the very preterm infant. Pediatr Res. 2014;75:564-9.
28. Huang J, Zhang L, Kang B, et al. Association
between perinatal hypoxic-ischemia and periventricular leuko-malacia
in preterm infants: A systematic review and meta-analysis. PLoS One.
2017;12:e0184993.
29. Tsimis ME, Johnson CT, Raghunathan RS, et al.
Risk factors for periventricular white matter injury in very low
birthweight neonates. Am J Obstet Gynecol. 2016;214: 380.e1-380.e6.
30. Hatzidaki E, Giahnakis E, Maraka S, et al.
Risk factors for periventricular leukomalacia. Acta Obstet Gynecol
Scand. 2009;88:110-5.
31. Shankaran S, Langer JC, Kazzi SN, et al.
Cumulative index of exposure to hypocarbia and hyperoxia as risk
factors for periventricular leukomalacia in low birth weight
infants. Pediatrics. 2006;118:1654-9.
32. Al-Abdi SY, Al-Aamri MA. A Systematic review
and meta-analysis of the timing of early intraventricular hemorrhage
in preterm neonates: Clinical and research implications. J Clin
Neonatol. 2014;3:76-88.
33. Dolfin T, Skidmore MB, Fong KW, et al.
Incidence, severity, and timing of subependymal and intraventricular
hemorrhages in preterm infants born in a perinatal unit as detected
by serial real-time ultrasound. Pediatrics. 1983;71:541-6.
34. Wu T, Wang Y, Xiong T, et al. Risk factors
for the deterioration of periventricular–intraventricular
hemorr-hage in preterm infants. Sci Rep. 2020;10:13609.
35. Inder TE, Anderson NJ, Spencer C, et al.
White matter injury in the premature infant: A comparison between
serial cranial sonographic and MR findings at term. AJNR Am J
Neuroradiol. 2003;24:805-9.
36. Miller SP, Cozzio CC, Goldstein RB, et al.
Comparing the diagnosis of white matter injury in premature newborns
with serial MR imaging and transfontanel ultrasonography findings.
AJNR Am J Neuroradiol. 2003;24:1661-9.
37. Horsch S, Skiold B, Hallberg B, et al. Cranial ultrasound and MRI
at term age in extremely preterm infants. Arch Dis Child Fetal Neonatal
Ed. 2010;95:F310-4.
.
|
|
|
 |
|

