|
|
|
Indian Pediatr 2021;58:
915-921 |
 |
Diagnostic
Accuracy of WINROP, CHOP-ROP and ROPScore in
Detecting Type 1 Retinopathy of Prematurity
|
|
Deena Thomas, 1 Shamnad Madathil,1
Anu Thukral,1
M Jeeva Sankar,1
Parijat Chandra,2
Ramesh Agarwal,1
Ashok Deorari1
From 1Division of Neonatology, Department of
Pediatrics, All India Institute of Medical Sciences;
and 2Department of Ophthalmology, Dr. Rajendra
Prasad Center for Ophthalmic Sciences; New Delhi.
Correspondence to: Dr Anu Thukral, Associate
Professor, Department of Pediatrics, All India
Institute of Medical Sciences, New Delhi 110029.
Email:
[email protected]
Received: November 06, 2020;
Initial review: December 27, 2020;
Accepted: May 12, 2021.
Published online: May 20, 2021;
PII: S097475591600328
|
|
Background: Algorithms for predicting
retinopathy of prematurity (ROP) requiring treatment
need to be validated in Indian settings to determine
if the burden of screening can be reduced without
compromising the sensitivity of existing gestation
and weight-based cut offs.
Objective:
To evaluate the performance of the available
algorithms namely, WINROP (Weight, Insulin-like
growth factor I, Neonatal ROP), CHOP-ROP (Children’s
Hospital of Philadelphia ROP) and ROPScore in
predicting type 1 ROP and time from alarm to
treatment by each algorithm.
Study design:
Ambispective observational.
Setting:
Tertiary care neonatal intensive care unit in India.
Participants:
Neonates less than 32 weeks or less than 1500 g
born between July, 2013 to June, 2019 (N=578),
who underwent ROP screening.
Primary outcome:
Sensitivity, specificity and time from alarm to
treatment by each algorithm.
Results: The
sensitivity and specificity of WINROP was 85% and
36%, for CHOP-ROP it was 54% and 71%, and for
ROPScore it was 73% and 67%, respectively in
detecting type 1 ROP. A total of 50/51 (98%) of
neonates with type 1 ROP underwent treatment at
median gestation of 9 weeks and median time from
alarm to treatment by WINROP, CHOP-ROP and ROPScore
was 7, 7 and 3 weeks, respectively.
Conclusion:
WINROP, CHOP-ROP and ROPScore were not sensitive
enough to replace the gestational age, weight and
risk factor-based screening criteria for type 1 ROP.
Keywords: Neonatal
intensive care unit, Premature, Sensitivity,
Specificity.
|
|
L ow- and
middle-income countries are currently facing the
third epidemic of retinopathy of prematurity (ROP)
on account of higher rate of preterm birth and wide
variations in neonatal care provided. Blencowe, et
al. [1] estimated that approximately 98077 neonates
in India would require screening for ROP amounting
to nearly three lakh examinations every year.
National guidelines recommend screening of all the
neonates <34 weeks or <2000 gram or neonates with
gestational age between 34-36 weeks with risk
factors for ROP such as prolonged oxygen support,
cardiovascular instability, and sepsis [2]. When
compared to screening criteria in developed
countries, these guidelines are much higher, as
bigger babies also develop severe ROP in developing
countries, and this further increases the screening
load [3,4]. Given the paucity of skilled
ophthalmologists for screening; gestation and
weight-based screening criteria increase the burden
on existing health systems, leading to poor quality
of services being provided and eventually leading to
missing out on cases requiring close follow up and
treatment.
Current conventional screening
method for ROP is a painful procedure. It leads to
physiological changes like hypertension and decrease
in oxygen saturation [5]. In addition, this is an
additional burden on the fragile health system. Many
screening algorithms have been developed and are in
place for more than a decade now. However, due to
their inability in providing 100% sensitivity
(assuming gestation and weight risk factor-based
screening criteria as standard), none of the
algorithms have been able to replace existing
protocols. These algorithms have shown high
sensitivity and negative predictive value in many
countries; however, they have not been widely
validated in Indian settings [6-8]. Due to lack of
sufficient literature in Indian settings, this study
was planned with the aim to evaluate the diagnostic
performance of all the three algorithms, namely
WINROP (Weight, Insulin-like growth factor I,
Neonatal ROP), CHOP-ROP (Children’s Hospital of
Philadelphia ROP) and ROPScore in predicting type 1
ROP in an Indian setting [9,10]. We also evaluated
time from alarm to treatment by each algorithm.
METHODS
This study was conducted as an
ambispective observational study with a
retrospective phase collecting data from 1 July,
2013 to 30 June, 2018 and a prospective phase
comprising of data collected from 1 July, 2018 to 30
June, 2019 at a tertiary care hospital. The policy
of our unit is to screen all neonates less than 32
weeks gestational age (GA) or neonates with a
birthweight less than 1500 g or bigger neonates
(32-34 weeks GA or bithweight 1501-2000 g) with risk
factors (respiratory or hemodynamic instability,
anemia requiring transfusion or culture positive
sepsis). Our unit has a strict pulse oximetry
monitoring policy for preterm infants care in the
NICU. Since only neonates less than 32 weeks GA can
be entered in WINROP and ROPScore, the neonates less
than 32 weeks or birthweight less than 1500 g who
underwent retinopathy of prematurity screening were
included in the study. Neonates with congenital
malformation, hydrocephalus and hydrops fetalis were
excluded.
Records of all the neonates who
underwent ROP screening in the retrospective phase
were retrieved from ROP registers maintained in the
unit. In addition, all the demographic details, and
antenatal, intrapartum and postnatal course details
were retrieved from the medical records department.
Birthweight, gestational age and weekly weight
(weight on postnatal day 8, 15, 22, 29 and so on) of
these infants till discharge was noted. Neonates on
invasive ventilation were weighed on alternate days
after disconnecting from ventilator for a brief
duration as per the unit policy. The appropriateness
of birthweight for gestational age was assigned by
the AIIMS intrauterine growth chart [11] for
neonates ³32
weeks of gestation or Lubchenco growth charts [12]
for neonates less than 32 weeks of gestation.
All the infants satisfying the
inclusion criteria were screened for ROP as per the
unit protocol at 4 weeks of postnatal age with the
exception of those <28 weeks whose first screen was
done at 2-3 weeks postnatal age. ROP was described
as per International Classification of Retinopathy
of Prematurity and was classified into treatment
group as per Early Treatment of Retinopathy of
Prematurity Classification [13,14]. The worst stage
of ROP and the presence of plus disease (when
present) was recorded. In cases where both eyes were
affected, worst stage of the ROP of either eye was
taken. Postnatal age of development of type 1 ROP as
defined by any ROP in Zone I with plus disease or
stage 3 ROP in zone I without plus disease or stage
2 or 3 ROP in Zone II with plus disease was noted
and the treatment provided was also recorded. The
infants with type 1 ROP findings who were lost to
follow up were contacted telephonically to know
their ophthalmological outcome and intervention done
(laser photocoagulation/anti-VEGF injection).
Similar data collection was performed for the
prospective phase after informed parental consent.
Ethical clearance was obtained from institute’s
ethics committee.
Data obtained from included
neonates was entered into the following three
predictive algorithms according to the eligibility
criteria:
WINROP: All the neonates less
than 32 weeks of gestation at birth irrespective of
the BW were eligible to be entered into WINROP,
which is available online (www.winrop.com)
[15]. Birthweight, gestational age and weekly weight
were entered till 35 weeks of postmenstrual age or
discharge, or till the alarm signals in the
algorithm, whichever was earlier. WINROP algorithm
requires that the weight of neonate be entered till
35 weeks of postmenstrual age (PMA) to classify a
neonate to be at low risk.
CHOP-ROP: Neonates less than
31 weeks of GA or less than 1501 g birthweight were
eligible to be evaluated by CHOP-ROP [16].
Birthweight, gestational age and daily weight gain
rate was entered into the algorithm to calculate the
risk score from 2nd week onwards. CHOP-ROP requires
documentation of neonatal weight at end of second
week to be included in the algorithm. Weight change
in the first week was disregarded as per the
original study. Daily weight gain rate was
calculated by weekly measurements (difference
between current weight and previous week’s weight
divided by 7). For neonates with gestation >28 week,
only birth weight and weight gain rate was used for
calculation. Alarm cutoff of
³0.010
was used to identify neonates at risk of type 1 ROP.
ROP score: Neonates
less than 32 weeks or <1500 g whose weight at end of
6th week postnatal age was available before
discharge or at follow up were eligible to be
included in the ROPScore algorithm proposed by
Eckert, et al. [17]. This score required data on use
of oxygen in mechanical ventilation (invasive or
non-invasive ventilation including CPAP upto sixth
completed week), requirement of blood transfusion up
to sixth completed week of life, neonate’s weight at
sixth completed week in addition to birthweight and
gestational age: ROPScore excel sheet was used for
calculation of the score. Cutoff for risk of type 1
ROP was taken as
³14.5.
Primary outcomes were to evaluate
the specificity and the sensitivity of three
screening algorithms namely, WINROP, CHOP-ROP and
ROPScore, in predicting type 1 ROP. Secondary
outcome was time from alarm to predict type 1 ROP by
these algorithms to the time the neonates underwent
treatment for the same.
The reported specificity for
CHOP-ROP was 51%, for ROPScore 57%, and for WINROP
was 60% [6-9,18]. To detect a similar magnitude of
difference (i.e. absolute difference of 9%) between
CHOP-ROP and WINROP algorithms, with a power of 80%
and alpha error of 5%, a total of 473 neonates had
to be enrolled.
Statistical analysis:
Statistical analysis was done using Stata 12.0
(StataCorp). Diagnostic performance of all the three
algorithms was described by calculating sensitivity,
specificity, positive predictive value, negative
predictive value, positive likelihood ratio,
negative likelihood ratio along with 95% confidence
interval for predicting the risk of type 1 ROP using
Open Epi ver 3.01. The receiver operating
characteristics (ROC) curve was constructed, and the
cutoff of ROPScore and CHOP-ROP with 100%
sensitivity and maximal specificity was calculated.
RESULTS
Out of 15,405 neonates born
during the study period with 898 neonates were less
than 32 weeks GA or birth weight <1500 g. The
records of 578 neonates who underwent at least one
ROP screening satisfying the inclusion criteria were
available. A total of 382 out of 578 (66%), 498 out
of 578 (86%) and 370 out of 578 (64%) neonates could
be analyzed for their risk of developing type 1 ROP
using WINROP, CHOP-ROP and ROPScore algorithms,
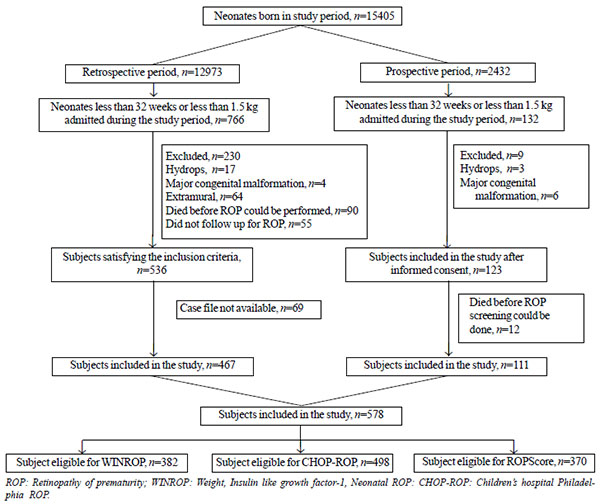
respectively. Fig. 1 describes the study flow
and reasons for exclusion from the study.
 |
|
Fig. 1 Study flow.
|
Neonates included in the study
had a mean (SD) GA and birth weight of 30.3 (2.4)
weeks and 1184 (308) gms, respectively. Other
demographic details have been provided in Table I.
One third of the neonates were noted to have any ROP
with a quarter of them requiring treatment (Table
II). No neonate less than 32 weeks having type 1
ROP was missed by the existing screening protocol;
amounting to sensitivity of 100% in this age group.
Around 70 (12%) neonates were lost to follow up from
the screening protocol out of which 5 neonates had
type 1 or 2 ROP on last screen available and were
contacted telephonically to know their final
ophthalmological outcome. All but one neonate with
type 1 ROP underwent treatment for the same at a
median postnatal age of 9 weeks or 36 weeks
postmenstrual age. Only one baby received anti- VEGF
injection during the study period.
Table I Baseline Characteristics of the Study Population (N=578)
| Characteristics |
Value |
| Gestational age (wk)a |
30.3 (2.37) |
| Birthweight (g)a |
1184 (308) |
| Small for gestational
age |
234 (40.5) |
| Male |
306 (52.9) |
| Singleton |
414 (71.6) |
| Complete antenatal
steroid coverage |
350 (60.5) |
| Resuscitation (more
than initial steps) |
181 (31.3) |
| Apgar score at 1 mina |
6.1 (2.02) |
| Apgar score at 5 mina |
7.5 (1.3) |
| Respiratory distress
requiring surfactant |
176 (30.4) |
| Bronchopulmonary
dysplasia |
81 (14) |
| Invasive ventilation |
150 (26) |
|
Invasive ventilation duration (d)b,c |
6 (3-19) |
|
Grade III or IV intraventricular hemorrhaged
|
18 (3.2) |
|
Periventricular leukomalaciad |
67 (11.6) |
|
Hemodynamically significant ductus
arteriosus
|
61 (10.5) |
|
Hypotension requiring inotropes |
60 (10.4) |
| Sepsis requiring
antibiotics |
182 (31.4) |
|
Day of regaining birth weighta
|
11.9 (5.3) |
| Anemia requiring
transfusion |
112 (19.4) |
| Data
expressed as no. (%) except amean (SD) or
bmedian (IQR). camong those who received it;
damong those screened. |
Table II Retinopathy of Prematurity in the Study Population
| Characteristics |
Retrospective |
Prospective |
Combined |
|
Cohort |
Cohort |
(n=578) |
|
(n=467) |
(N=111) |
|
| Any ROP |
183 (39.2) |
25 (22.5) |
208 (36) |
| Type of ROP |
|
|
|
| Type 1 |
42 (8.9) |
9 (8.1) |
51 (8.8) |
| Type 2 |
18 (3.8) |
1 (0.9) |
19 (3.3) |
| Mild ROP |
123 (26.3) |
15 (13.5) |
138 (23.9) |
|
Identification of any ROP (wk)a,b |
6 (4-8) |
7 (6-9) |
6 (4-8) |
|
Identification of type1 ROP (wk)a,b |
9 (7-10) |
9 (7-12) |
9 (7-10) |
|
Number of screeningsa |
3 (2-5) |
3 (2-4) |
3 (2-5) |
| Data
represented as n (%) or amedian (IQR). btime
to identification. ROP-retinopathy of
prematurity. |
Table III Diagnostic Performance of WINROP, CHOP-ROP and ROPScore
| Parameter |
WINROP |
CHOP-ROP |
ROPScore |
|
(n=382) |
(n=498) |
(n=370) |
| Sensitivity (%) |
85.4 |
54 |
72.9 |
|
(72.8-92.7) |
(40.4-67.0) |
(59-83.4) |
| Specificity (%) |
36.2 |
71.4 |
67.3 |
|
(31.3-41.5) |
(67.1-75.4) |
(61.9-2.2) |
| PPV (%) |
16.1 |
17.4 |
25 |
|
(12.1-21.2) |
(12.3-24.2) |
(18.6-32.8) |
| NPV (%) |
94.5 |
93.3 |
94.3 |
|
(89.1-97.3) |
(90.1-95.5) |
(90.5-96.6) |
| Positive LR |
1.3 |
1.9 |
2.3 |
|
(1.3-1.4) |
(1.7-2.0) |
(2.1-2.3) |
| Negative LR |
0.4 |
0.6 |
0.4 |
|
(0.3-0.5) |
(0.6-0.7) |
(0.3-0.5) |
| Diagnostic OR |
3.3 |
2.9 |
5.5 |
|
(1.4-7.6) |
(1.6-5.3) |
(2.8-10.9) |
| NNS |
9.4 (5.9-21.4) |
9.6 (6.2-21.1) |
5.2 (3.8-7.9) |
| 95% CI in
parenthesis. ROP-retinopathy of prematurity;
WINROP-weight, insulin-like growth factor I,
neonatal, ROP; CHOP-ROP- Children’s Hospital
of Philadelphia ROP; PPV-Positive predictive
value; NPV-Negative predictive value; LR-
likelihood ratio; OR-odds ratio; NNS-Number
needed to screen. |
Diagnostic performance of the
three screening algorithms has been provided in
Table III. WINROP had the maximum sensitivity
(85%) to identify neonates with type 1 ROP followed
by ROPScore and then CHOP-ROP. Specificity followed
the reverse order with CHOP-ROP being most specific
(71%). Decreasing the cutoff point of ROPScore to
10.79 gave 100% sensitivity with a specificity of
16.5% (12.8%-20.9%) and avoided screening in 61
neonates. WINROP and CHOP-ROP identified type 1 ROP
earliest at 2 weeks of postnatal age, around 7 weeks
before conventional screening method where the
neonates with type 1 ROP were identified and treated
at 9 weeks of postnatal age. ROPScore identified
neonates at risk of type of type 1 ROP at 6 weeks of
postnatal age, by which time 3 neonates were already
treated for type 1 ROP by conventional screening
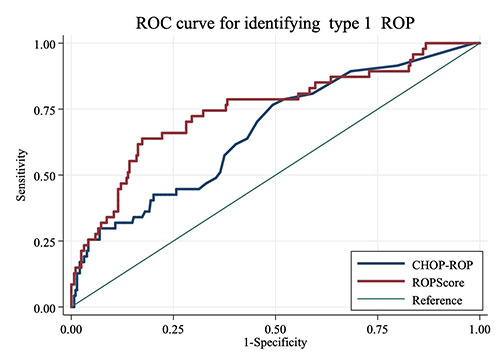
method. ROC curve of CHOP-ROP and ROPScore for
identifying type 1 ROP among 334 neonates showed
area under curve of ROPScore [0.75 (0.66-0.83)] to
be more than that of CHOP-ROP [0.66 (0.58-0.95)] (Fig.
2). Since WINROP gives only binary output to
signify the risk of developing type 1 ROP unlike a
continuum of scores provided by CHOP-ROP and
ROPScore, an ROC curve for the same was not
constructed.
 |
|
ROC: Receiver operating characteristics
curve; CHOP-ROP- Children’s Hospital of
Philadelphia ROP; ROP-retinopathy of
prematurity.
Fig. 2 ROC curve of CHOP-ROP and
ROPScore for identifying type 1 ROP.
|
DISCUSSION
The study was conducted at a
level III neonatal intensive care unit on intramural
neonates. The unit caters mainly to high risk
neonates who are referred in utero from many parts
of North India early in gestation and where gentle
ventilation guided by pulse oximetry along with
antibiotic stewardship is the norm.
Our rates of ROP and type I ROP
were higher than the literature [19], possibly due
to the smaller gestational age and lesser
birthweight of our neonates. Sensitivity of WINROP
in our cohort was 85.42% which was slightly lower
than the recent study by Sanghi, et al. [10] (90%).
Low sensitivity (65%) of WINROP was observed in a
study in Taiwan where older and larger neonates
developed ROP requiring treatment which were missed
by the WINROP [20]. The specificity (36%), positive
predictive value (16%) and high negative predictive
value (94%) in our study was in accordance with the
previously reported literature [8,21,22].
CHOP-ROP performed poorly in our
cohort with a sensitivity of 54%. This was lower
than that reported by Doshi, et al. [9] (67%) in
2019 Indian infants in spite of their cohort dealing
with bigger neonates. They used the nomogram
provided by Binenbaum, et al. [16] for manual
calculation of alarm limit. This method was not
considered feasible in our setting due to large
sample size and hence the original formula provided
by Binenbaum, et al. [16] was used. In the study by
Doshi, et al. [9] decreasing the cutoff from 0.014
to 0.010 gave 100% sensitivity. However, in our
study the cutoff had to be decreased to 0.001 to
give 100% sensitivity, which in turn decreased the
specificity to unacceptable levels (2.23%).
The sensitivity of ROPScore was
73% which was lower than previous studies (95-100%)
[6,23]. When the cutoff of ROPScore was decreased to
10.79, the sensitivity approached 100% and this cut
off potentially would avoid screening in 16.5% of
neonates and thus has clinical implication. ROPScore
showed better diagnostic performance with an area
under curve of 0.75 vs 0.66 of CHOP-ROP. However,
ROPScore has inherent disadvan-tages as it gives an
alarm at 6 weeks of postnatal age when most of the
neonates with aggressive posterior ROP are already
identified by conventional screening methods and
treated. In addition, many neonates with risk
factors who are discharged before six weeks of
postnatal age cannot be evaluated using ROPScore
thereby missing out on cases with type 1 ROP.
The median time from alarm to
treatment in our study for WINROP, CHOP-ROP and
ROPScore was 7, 7 and 3 weeks, respectively which
was lower than those previously estimated [24],
where it was 11.1, 9.1 and 5.1 week, respectively.
An ideal algorithm for
identifying type 1 ROP is the one with 100%
sensitivity and a reasonable level of specificity so
as to reduce the unwanted ROP screenings being done
currently. None of the algorithms were sensitive
enough in our setting probably due to a higher
saturation target of 90-95% being followed in the
unit. A similar decrease in sensitivity of WINROP
from 87.5% to 48% was noted by Lundgren, et al. [25]
when the saturation targets increased from 88-92% in
2011-2012 to 91-95% in 2015-2016.
Strengths of our study are its
large sample size, and using registers maintained by
the staff and doctors of the unit containing data of
neonates who underwent ROP screening to retrieve the
files of neonates who underwent screening, and this
was cross-checked with the electronic discharge data
of the unit. Three rounds of file retrieval from
medical records department was conducted before
classifying a file as non-available. Our study has
some limitations as well. The weight was not
available at 6 weeks completed age in 196 out of 467
(42%) neonates enrolled in retrospective phase. None
of the algorithms could accommodate all the neonates
included in the study, thereby true comparison of
diagnostic performance of the various algorithms
with the existing weight and gestation-based
criteria could not be performed.
In conclusion, none of the
screening algorithms with their recommended cutoffs
was able to provide 100% sensitivity as provided by
the weight, gestational age and risk factor-based
screening protocol being currently followed in the
unit. Although ROPScore with a modified cutoff of
10.79 looks promising since it has 100% sensitivity,
it has a poor specificity of 16.5% and it gives an
alarm at 6 weeks completed age, a time at which few
of the neonates would already have been identified
by conventional screening method.
Ethics clearance:
Institutional ethics committee of Post Graduate
research (clinical sciences), AIIMS, New Delhi; No.
IECPG-280 dated 28 June, 2018.
Contributors: DT: prepared
the first draft of the protocol and had the prime
responsibility of data collection, data analysis and
compilation of results; SM: collected data, cross
checked data entry and contributed to the
manuscript; AT: conceptualized the study, supervised
data entry and provided input in preparation of
protocol and final manuscript; MJS: contributed to
protocol formation, helped in statistical analysis
and contributed to final manuscript; PC: valuable
suggestion during protocol formation and provided
input to final manuscript; RA: critically reviewed
the protocol of the study, ensured timely progress
of the study via departmental meetings and provided
input to final manuscript; AD: input in protocol of
the study and critically reviewed the final
manuscript. All the authors in principal agreed to
the final manuscript of the study.
Funding: None; Competing
interest: None stated.
|
WHAT IS ALREADY KNOWN?
• Gestational age, weight
based as well as risk factor-based criteria
are generally followed to screen neonates at
risk for developing type 1 ROP.
WHAT THIS STUDY ADDS?
• None of the three
screening algorithms examined in the study
was able to provide 100% sensitivity as
provided by the weight, gestational age and
risk factor-based screening protocol.
|
REFERENCES
1. Blencowe H, Moxon S, Gilbert
C. Update on blindness due to retinopathy of
prematurity globally and in India. Indian Pediatr.
2016;53:S89-92.
2. Shukla R, Murthy GVS, Gilbert
C, et al. Operational guidelines for ROP in India: A
Summary. Indian J Ophthalmol. 2020;68:S108-14.
3. Fierson WM; American Academy
of Pediatrics Section on Ophthalmology; American
Academy of Ophthalmology; American Association for
Pediatric Ophthalmology and Strabismus; American
Association of Certified Orthoptists. Screening
Examination of Premature Infants for Retinopathy of
Prematurity. Pediatrics. 2018;142: e20183061.
4. Shah PK, Narendran V, Kalpana
N, et al. Severe retinopathy of prematurity in big
babies in India: History repeating itself? Indian J
Pediatr. 2009;76:801-4.
5. Jiang J-B, Zhang Z-W, Zhang
J-W, et al. Systemic changes and adverse effects
induced by retinopathy of prematurity screening. Int
J Ophthalmol. 2016;9:1148-55.
6. Cagliari PZ, Lucas VC, Borba
IC, et al. Validation of ROPScore to predict
retinopathy of prematurity among very low birth
weight preterm infants in a southern Brazilian
population. Arq Bras Oftalmol. 2019;82:476-80.
7. Timkovic J, Pokryvkova M,
Janurova K, et al. Evaluation of the WinROP system
for identifying retinopathy of prematurity in Czech
preterm infants. Biomed Pap Med Fac Univ Palacky
Olomouc Czechoslov. 2017;161:111-6.
8. Jung JL, Wagner BD, McCourt EA
et al. Validation of WINROP for detecting
retinopathy of prematurity in a North American
cohort of preterm infants. J AAPOS. 2017;21:229-233.
9. Doshi S, Desai S, Nanavati R,
et al. Children’s hospital of Philadelphia Score to
predict severe retinopathy in Indian preterm
infants. Eye. 2019;33:1452-8.
10. Sanghi G, Narang A, Narula S,
et al. WINROP algorithm for prediction of sight
threatening retinopathy of prematurity: Initial
experience in Indian preterm infants. Indian J
Ophthalmol. 2018;66:110-3.
11. Singh M, Giri SK,
Ramachandran K. Intrauterine growth curves of live
born single babies. Indian Pediatr. 1974;11:475-9.
12. Lubchenco LO, Hansman C,
Dressler M, Boyd E. Intrauterine growth as estimated
from liveborn birth-weight data at 24 to 42 weeks of
gestation. Pediatrics. 1963; 32:793.
13. International Committee for
the Classification of Retinopathy of Prematurity.
The International Classification of Retinopathy of
Prematurity revisited. Arch Ophthalmol.
2005;123:991-9.
14. Hardy RJ, Good WV, Dobson V,
et al. The early treatment for retinopathy of
prematurity clinical trial: Presentation by
subgroups versus analysis within subgroups. Br J
Ophthalmol. 2006;90:1341-2.
15. Löfqvist C, Andersson E,
Sigurdsson J, et al. Longitudinal postnatal weight
and insulin-like growth factor 1 measurements in the
prediction of retinopathy of prematurity. Arch
Ophthalmol. 2006;124:1711-8.
16. Binenbaum G, Ying G, Quinn
GE, et al. The CHOP postnatal weight gain, birth
weight, and gestational age retinopathy of
prematurity risk model. Arch Ophthalmol. 2012;130:
1560-5.
17. Eckert GU, Fortes Filho JB,
Maia M, et al. A predictive score for retinopathy of
prematurity in very low birth weight preterm
infants. Eye. 2012;26:400-6.
18. Ali E, Al-Shafouri N, Hussain
A, et al. Assessment of WINROP algorithm as
screening tool for preterm infants in Manitoba to
detect retinopathy of prematurity. Paediatr Child
Health. 2017;22:203-6.
19. Kumar P, Sankar MJ, Deorari
A, et al. Risk factors for severe retinopathy of
prematurity in preterm low birth weight neonates.
Indian J Pediatr. 2011;78:812-6.
20. Ko C-H, Kuo H-K, Chen C-C, et
al. Using WINROP as an adjuvant screening tool for
retinopathy of prematurity in Southern Taiwan. Am J
Perinatol. 2015;30:149-54.
21. Sun H, Kang W, Cheng X, et
al. The use of the WINROP screening algorithm for
the prediction of retinopathy of prematurity in a
Chinese population. Neonatology. 2013;104:127-32.
22. Piyasena C, Dhaliwal C,
Russell H, et al. Prediction of severe retinopathy
of prematurity using the WINROP algorithm in a birth
cohort in South East Scotland. Arch Dis Child Fetal
Neonatal Ed. 2014;99:F29-33.
23. Lucio KCDV, Bentlin MR,
Augusto ACL, et al. The ROPScore as a screening
algorithm for predicting retinopathy of prematurity
in a Brazilian population. Clinics (Sao Paulo).
2018;73:e377.
24. Piermarocchi S, Bini S,
Martini F, et al. Predictive algorithms for early
detection of retinopathy of prematurity. Acta
Ophthalmol. 2017;95:158-164.
25. Lundgren P, Hård AL, Wilde Å
et al. Implementing higher oxygen saturation targets
reduced the impact of poor weight gain as a
predictor for retinopathy of prematurity. Acta
Paediatr. 2018;107:767-773.
|
|
|
 |
|

