|
|
|
Indian Pediatr 2019;56:
873-875 |
 |
School-age Children as Asymptomatic Malaria Reservoir in
Tribal Villages of Bastar Region, Chhattisgarh
|
|
R Ranjha 1*,
GDP Dutta1 and SV
Gitte2
From 1ICMR-National Institute of
Malaria Research,
FU-Raipur; and 2Regional Office of Health and Family Welfare and
Regional Leprosy training and Research Institute, Raipur; Chhattisgarh,
India.
*[email protected]
|
|
Malaria is a major health concern in India, especially in regions
populated by tribals. In this cross-sectional survey carried out in
Bastar region of Chhattisgarh, 35 Plasmodium infections were detected in
451 participants screened during the non-transmission season; 27 (77.1%)
were asymptomatic. Participants with age 6-14 years were at high risk of
asymptomatic infection [OR 4.09, 95% CI, 1.69 to 9.89, P=0.001],
and may act as an under-appreciated reservoir for sustained malaria
transmission.
Keywords: Control,
Diagnosis, Plasmodium, Management, Transmission.
|
|
I
ndia has a target of malaria elimination by 2030.
Controlling and elimination of malaria from tribal communities is a
major task and need more attention to achieve the target of malaria
elimination [1]. Tribal populations are reported to have naturally
acquired immunity to malaria [2]. Due to this immunity, individuals do
not develop clinical symptoms and do not seek medical treatment.
School-age children and adults that are not the main focus of malaria
prevention strategies may act as reservoirs for malaria transmission due
to the naturally acquired immunity [3]. Tribals constitute more than 30%
of the total population of Chhattisgarh. To control and eliminate
malaria from Chhattisgarh it will be important to identify the potential
reservoir for malaria transmission. This study was undertaken to find
out the reservoir of asymptomatic malaria in the tribal population of
Bastar region, Chhattisgarh, just before the transmission season in July
2017.
The study was carried out in the Keshkal block,
Kondagaon district, Bastar division, Chhattisgarh. Using Epiinfo 7
software, a sample size of 422 was calculated with expected frequency 7%
[5], design effect 1.5 and 99.9% confidence interval. Five villages were
randomly selected for the survey out of 101 villages in the block.
Households from these villages were selected using systematic sampling,
and blood sample was collected from every member of household who was
eligible and had given consent. Blood samples were collected from 451
participants. Written informed consent was obtained from all the
participants and the guardian of minors participating in the study. This
study was approved by the institutional ethics committee, ICMR-National
Institute of Malaria Research.
A short clinical assessment of all the study
participants was done and information related to malaria-related
symptoms (fever, headache, vomiting, and nausea) was recorded. Malaria
diagnosis was performed using microscopy. Blood slides were stained with
JSB stain and examined under compound microscope (Carl Zeiss Oberkochen,
Germany) at 100X magnification for malaria parasite detection. Diagnosis
was done by two trained laboratory technicians. Both thick and thin
smears were made on the microscopic slides. Thick smears were used for
parasite detection and thin smears were used for species identification.
Statistical analysis was performed using SPSS version 20.0 (Armonk, New
York, USA) and GraphPad Prism software (La Jolla, CA 92037 USA). Student
t-test and chi-square test were used. P-value <0.05 was considered as
significant.
TABLE I Age- and Gender-wise Distribution of Study Participants
|
Variable |
Total participants |
Malaria negative |
Asymptomatic malaria
|
Symptomatic malaria
|
|
(n=451) |
(n=416) |
positive (n=27) |
positive (n=8) |
|
Male sex, n (%) |
174 (38.6) |
158 (38.0) |
13 (48.1) |
3 (37.5)
|
|
Age (y)* |
23.4 (16.7)
|
24.0 (16.8)
|
13.4 (8.9)
|
23.6 (19.7)
|
|
Age distribution, n (%) |
|
6 mo-5 y |
26 (5.8) |
24 (5.8) |
2 (7.4) |
0 |
|
6-14 y |
75 (16.6) |
53 (12.7) |
18 (66.7) |
4 (50) |
|
³15 y |
350 (77.6) |
339 (81.5) |
7 (25.9) |
4 (50) |
Mean age (range) of participants was 19 (1-71) years
(61.8% females) (Table I). A total of 35 (7.8%) malaria
cases were detected in the surveyed population; 94.3% were by
Plasmodium falciparum and the rest were the mixed infection with
P. vivax. Of these, 77.1% of the cases were asymptomatic. Two-thirds
(66.7%) of asymptomatic patients belonged to school-age group (6-14
years) (Table I). Mean age of participants with
asymptomatic malaria was significantly lower than the symptomatic cases
and non-malarial participants (Fig. 1). Asymptomatic
malaria showed association with age, and no association was observed
with gender. Risk of asymptomatic malaria was high in participants with
age £14 years
(OR 4.09, 95% CI 1.69 to 9.89, P=0.001).
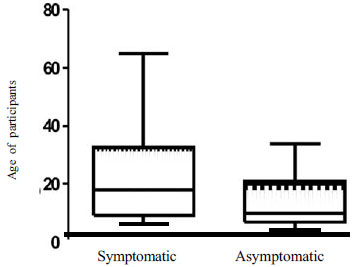 |
|
Fig.1. Box-and-whisker plot showing
age-distribution among symptomatic and asymptomatic malaria
cases.
|
Controlling malaria in tribal populations requires
more effort and is of immense importance to achieve the target of
malaria elimination in the country. The malaria transmission season in
Chhattisgarh starts from August with the peak in October-November, the
asymptomatic reservoir present in the population in July-August may act
as a very important contributing factor for increased transmission in
the coming months. This is the time when vector density increases,
leading to high transmission in the following months. As microscopy is
the method available at Primary Health Center level, we used it to find
out the asymptomatic cases in monsoon season i.e., July end.
Asymptomatic malaria cases were reported to be five
times the clinical cases of malaria in low transmission season in
central Malli, West Africa [4]. 6.8% of the population was an
asymptomatic carrier of infection in eastern India [5]. Chaurasia, et
al. [6] reported that 77.7% of malaria infections were asymptomatic.
In our study, we found that 6% of the population was carrying
asymptomatic malarial infection in low transmission season.
Alves, et al. [7] previously reported an
association of asymptomatic malaria with older age group [7], whereas
previous Indian authors found a high prevalence of afebrile parasitemia
in younger individuals (<14 years) [8,9]. Aju-Ameh, et al. [10]
also showed a prevalence of asymptomatic malaria in the age group 2-10
years. Prevalence of asymptomatic malaria was reported to be higher in
age group >15 years in Chhattisgarh [6]. Our study contradicts findings
of Alves, et al. [7] and Chaurasia, et al. [6] and
supports the earlier reported observations [8-10].
The results of this study indicate that for malaria
control and elimination within the set time frame, strategies should be
designed to find out and target the reservoir of asymptomatic malaria
before the rainy season. This may show a significant reduction in number
of malaria cases in high transmission season. This strategy may be
highly effective for bringing areas in control phase of malaria to
pre-elimination phase. The limitation of this study is the small sample
size. Studies with a large sample size and at multiple sites are
warranted to better understand the problem of asymptomatic reservoir in
Chhattisgarh, and other areas with high transmission of malaria.
Contributors: DG, GS: conceptualized and
designed the study; DG: collected the data; RR: analyzed the data and
drafted the manuscript. All authors approved the final version of
manuscript, and agree to be accountable for all aspects of study.
Funding: None; Competing interest: None
stated.
References
1. National Framework for Malaria Elemination in
India (2016-2030). (Feb 3, 2016). National Vector Borne Disease Control
Program, Government of India. Available from:http://www.who.int/iris/handle/10665/246096.
Accessed November 5, 2018.
2. Das MK, Prajapati BK, Tiendrebeogo RW, Ranjan K,
Adu B, Srivastava A, et al. Malaria epidemiology in an area of
stable transmission in tribal population of Jharkhand, India. Malar J.
2017;16:181.
3. Walldorf JA, Cohee LM, Coalson JE, Bauleni A,
Nkanaunena K, Kapito-Tembo A, et al. School-age children are a
reservoir of malaria infection in Malawi. PLoS One. 2015;10:e0134061.
4. Coulibaly D, Travassos MA, Tolo Y, Laurens MB,
Kone AK, Traore K, et al. Spatio-temporal dynamics of
asymptomatic malaria: Bridging the gap between annual malaria
resurgences in a Sahelian environment. Am J Trop Med Hyg.
2017;97:1761-9.
5. Ganguly S, Saha P, Guha SK, Biswas A, Das S, Kundu
PK, et al. High prevalence of asymptomatic malaria in a tribal
population in eastern India. J Clin Microbiol. 2013;51:1439-44.
6. Chourasia MK, Raghavendra K, Bhatt RM, Swain DK,
Meshram HM, Meshram JK, et al. Additional burden of asymptomatic
and sub-patent malaria infections during low transmission season in
forested tribal villages in Chhattisgarh, India. Malar J. 2017;16:320.
7. Alves FP, Durlacher RR, Menezes MJ, Krieger H,
Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax
and Plasmodium falciparum infections in native Amazonian populations. Am
J Trop Med Hyg. 2002;66:641-8.
8. Chaturvedi N, Krishna S, Bharti PK, Gaur D,
Chauhan VS, Singh N. Prevalence of afebrile parasitaemia due to
Plasmodium falciparum and P. vivax in district Balaghat (Madhya
Pradesh): Implication for malaria control. Indian J Med Res.
2017;146:260-6.
9. Chourasia MK, Raghavendra K, Bhatt RM, Swain DK,
Valecha N, Kleinschmidt I. Burden of asymptomatic malaria among a tribal
population in a forested village of central India: A hidden challenge
for malaria control in India. Public Health. 2017;147:92-7.
10. Aju-Ameh C. Prevalence of asymptomatic malaria in
selected communities in Benue state, North Central Nigeria: A silent
threat to the national elimination goal 2017. Edorium J Epidemiol.
2017;3:1-7.
|
|
|
 |
|

