|
|
|
Indian Pediatr 2019;56: 841-844 |
 |
Comparison of Soybean-based Oil and
MCT-olive-fish-soy Oil Intravenous Lipid Emulsions on Soluble
Adhesion Markers in Preterm Neonates with Sepsis: A
Randomized Controlled Trial
|
|
Mohammed Abdelkareem 1,
Yahya Wahba1,
Basma Shouman1
and Abeer Mesbah2
From Departments of 1Pediatrics and 2Clinical
Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
Correspondence to: Dr Yahya Wahba, Assistant
Professor, Department of Pediatrics, Faculty of Medicine, Mansoura
University, 35516, Mansoura, Egypt,
Email:
[email protected]
Received: October 26, 2018;
Initial review: April 15, 2019;
Accepted: August 1, 2019.
Published online: August 10, 2019.
PII:S097475591600134
|
|
Objectives: To compare the
effects of two different intravenous lipid emulsions on soluble adhesion
markers in preterm infants with sepsis. Methods: This randomized
controlled pilot trial was conducted from February 2016 to February
2017. 40 preterm infants with sepsis were enrolled and assigned to
receive either Medium chain triglyceride-Olive-Fish-Soy lipid emulsion
(MOFS-LE) or soybean oil-based lipid emulsion (S-LE). Outcomes of the
study were changes in sICAM-1 and leukocyte integrin
b2
levels, and growth after 7 days of intervention. Results:
Leukocyte integrin b2
was significantly higher in MOFS-LE group. No statistically significant
differences were observed for sICAM-1, duration of mechanical
ventilation and antibiotics treatment, and mortality rate.
Conclusions: Leukocyte integrin
b2 was significantly higher in
preterm septic neonates who received MOFS-LE.
Keywords: Parenteral
nutrition, Polyunsaturated fatty acids, Soluble adhesion molecules.
Trial registration: ClinicalTrials.gov
(NCT03275090)
|
|
P
reterm infants with sepsis are more vulnerable to
undernutrition [1]. Adequate parenteral nutrition (PN) minimizes weight
loss, mortality and improves neurodevelopment [2]. Lipid emulsions (LEs)
are important components of PN providing energy, essential fatty acids
and vitamins [3].
Pure soybean oil-based lipid emulsions (S-LE) have
been used commercially worldwide, and consist of long-chain fatty acids
with omega 3 to omega 6 ratio of 1:5.5 [4]. These fatty acids play an
important role in several physiological processes such as immune and
inflammatory response, platelet functions and early neural and visual
development [5]. However, there are several studies suggesting that S-LE
could have hazards due to excess linoleic acid and polyunsaturated fatty
acids [6]. MOFS (MCT-olive-fish-soy oil) lipid emulsions (MOFS-LE) are
mixtures of 30% medium chain triglycerides (MCT), 25% olive oil, 15%
fish oil, and 30% soybean oil, and are supposed to have better immune-modulatory
and anti-inflammatory properties [3].
Quite a few studies comparing efficacy and safety of
S-LE and MOFS-LE in neonates exist but there is no consensus on the
ideal LE [3]. The present study was designed to compare short-term
effects of S-LE and MOFS-LE on soluble adhesion markers of sepsis and
growth in preterm infants.
Methods
This randomized controlled double-blind pilot trial
was conducted in a university-affiliated neonatal care unit (NCU) of
Mansoura (Egypt) from February 2016 to February 2017. The study was
approved by Medical Research Ethical Committee of Medical Faculty of
Mansoura University.
Preterm neonates with possible sepsis who had
positive isolates on blood culture were considered eligible for
inclusion. Neonates with major congenital malformations, inborn errors
of metabolism, hypoxic-ischemic encephalopathy and congenital infections
were excluded. Neonates were enrolled after obtaining written informed
consent from their legal guardians.
A fixed block randomization (4 per block) was used to
generate the sequence. Opaque sealed envelopes were used for allocation
concealment. The study medications were dispensed in identical appearing
coded bottles from the outpatient department pharmacy. Participants and
care providers were all blinded to the intervention. The randomization
sequence and the key to the code on the medication bottles were kept
with a researcher who was not involved in patient enrolment, medication
administration or measuring outcomes. Patients were randomized to
receive parenteral nutrition containing MOFS-LE or S-LE (Smoflipid, 20%
Intralipid respectively, Fresenius Kabi, Uppsala, Sweden) for 7 days. PN
was prepared aseptically by a special NCU nurse. The initial dose of LE
was 0.5 g/kg/day on first day of PN, increased to a maximum of 3.5
g/kg/day. When serum triglyceride level exceeded 250 mg/dL, the lipid
dosage was reduced by 25%. Amino acids, carbo-hydrates, trace elements,
vitamins and electrolytes were prescribed in both groups. Neonates with
early-onset sepsis received ampicillin and gentamicin while those with
late-onset sepsis received flucloxacillin with either cefotaxime or
gentamicin.
In all neonates, descriptive clinical data were
collected at enrollment and growth data till 7 days post-randomization.
Laboratory workup included complete blood count (CBC), C-reactive
protein (CRP), blood culture using BACTEC 9120 culture system, serum
creatinine, serum triglyceride, and blood glucose. Soluble intercellular
adhesion molecule 1 (sICAM-1) and leukocyte integrin ß2 were measured by
ELISA.
Primary outcomes were changes in levels of sICAM-1
and leukocyte integrin b2
after seven days of receiving the lipid emulsions. Secondary outcomes
included changes in growth parameters (weight, length and head
circumference), duration of mechanical ventilation and antibiotics
treatment, and mortality. A convenience sample of 40 neonates was
planned for this study.
Statistical analysis: Chi-square and Fisher exact
tests were used for categorical variables. Between-groups comparisons
were done using Mann–Whitney and Student’s t-tests. Within-group
comparisons were done by Wilcoxon and paired t-tests. An intention to
treat analysis was done. SPSS version 22 was used for statistical
analyses.
Results
Ninety preterm infants were assessed for eligibility;
50 were excluded and 40 infants with sepsis were included (Fig.
1). Both groups were comparable for baseline characteristics (Table
I). They were also comparable for the hematological parameters
and CRP. Klebsiella was the commonest organism followed by
Staphylococcus aureus and E. coli (n=12, 2 and 2,
respectively in MOFS-LE group; and n=9, 7 and 2, respectively in
S-LE group). Group B streptococcus and candida were isolated in two
patients each in MOFS-LE group; and pseudomonas was isolated in two
patients of S-LE group.
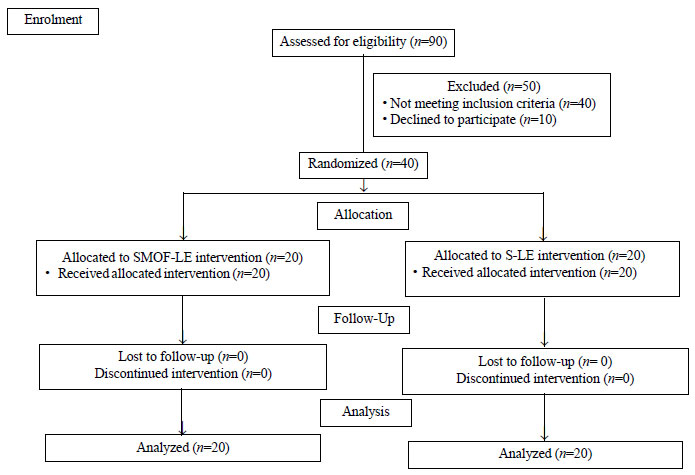 |
|
Fig. 1. CONSORT flow diagram of the
trial.
|
TABLE I Baseline Characteristics of Study Groups
|
Characters |
S-LE group |
MOFS-LE group
|
|
|
(n=20) |
(n=20) |
|
Gestational age (wk)* |
31.7 (2.0) |
31.6 (1.5) |
|
Male sex |
14 (70) |
8 (40) |
|
Growth status |
|
|
|
AGA |
13 (65) |
16 (80) |
|
SGA |
6 (30) |
4 (20) |
|
LGA |
1 (5) |
0 |
|
Onset of sepsis |
|
Early onset |
12 (60) |
8 (40) |
|
Late onset |
8 (40) |
12 (60) |
|
Maternal age (y)* |
27.3 (5) |
27 (5.4) |
|
Cesarean delivery |
18 (90) |
15 (75) |
|
Risk factors for sepsis |
|
PROM |
13 (65)
|
18 (90) |
|
Maternal UTI |
10 (50) |
7 (35)
|
|
Maternal fever
|
7 (35) |
7 (35) |
|
Chorioamnionitis |
4 (20) |
5 (25) |
|
Central line insertion |
7 (35)
|
3 (15) |
|
Surgery
|
0 |
2 (10) |
|
Data are shown as number (%), except *mean (SD); AGA:
Appropriate for gestational age; SGA: Small for gestational age;
LGA: Large for gestational age; PROM: Prolonged rupture of
membranes; UTI: Urinary tract infection; S-LE: soybean oil-based
lipid emulsion; MOFS-LE: Medium chain
triglyceride-Olive-Fish-Soy lipid emulsion. |
TABLE II Outcome Variables Between the Groups
|
Parameters |
S-LE group |
MOFS-LE group |
P value
|
|
sICAM-1 (pg/mL)#
|
(n=20) |
(n=20) |
|
|
Day 1 |
1214 (951.2-1658.5) |
1661.5 (819.4-2225.5)
|
0.02
|
|
Day 7 |
1493 (806.6-2018.7) |
2354 (1517.6-2902.1) |
0.02 |
|
Leukocyte integrin b2 (pg/mL)#
|
|
Day 1 |
19.5 (15.3-24.5) |
20 (14.3-24.4) |
0.41 |
|
Day 7 |
20.3 (15.2-25.6) |
25 (22.8-29.7) |
0.02 |
|
Weight (kg) #
|
|
Day 1 |
1.35 (0.8-1.9) |
1.4 (0.7-1.6) |
0.89 |
|
Day 7 |
1.4 (1.1-2.9)
|
1.5 (1.0-1.8)
|
0.92 |
|
Length (cm)*
|
|
Day 1
|
40.3 (4) |
39.1 (3.2) |
0.30 |
|
Day 7 |
40.4 (4.1) |
39.4 (3.1) |
0.43 |
|
Head circumference (cm)*
|
|
Day 1
|
29.1 (3.6) |
29.2 (3.4) |
0.90 |
|
Day 7 |
29.6 (3.4) |
29.9 (3.9) |
0.67 |
|
sICAM-1: Soluble intercellular adhesion molecule 1; Data are
shown as *mean (SD) or #median (IQR); S-LE: soybean oil-based
lipid emulsion; MOFS-LE: Medium chain
triglyceride-Olive-Fish-Soy lipid emulsion. |
Table II compares the outcomes of the
study between the two groups. sICAM1 and leukocyte integrin
b2 were significantly
higher in MOFS-LE group on 7th
day. Within-group comparison showed that sICAM-1 increased significantly
in both groups from 1st to 7th
day (P=0.030 in S-LE group and P=0.001 in MOFS-LE group)
while leukocyte integrin b2
increased significantly in MOFS-LE group only (P=0.001). For the
growth outcomes, within-group comparison revealed significant body
weight increase in both groups (P=0.010 in S-LE group and P=0.005
in MOFS-LE group). Length increased significantly in MOFS-LE group (P=0.004).
No significant differences were observed between both groups as regards
mortality, duration of mechanical ventilation or antibiotics treatment.
Discussion
In the present study there was no evidence of any
significant effect of the intervention in the levels of sICAM-1, while
leukocyte integrin b2
was significantly higher in the MOFS-LE group. There was no evidence of
any significant effect of the intervention on the growth parameters of
weight, length and head circumference between both groups.
Several soluble adhesion molecules are released in
sepsis. Among these, sICAM-1 and leukocyte integrin
b2 are early
predictors with high specificity and sensitivity, but are important in
controlling the infective process [7]. In the present study the
increased level of sICAM-1 in both groups is similar to that observed by
Edgar, et al. [8]. Briassoulis, et al. [9] reported that
septic infants with the highest sICAM-1 levels had better outcomes. The
significantly higher levels of leukocyte integrin
b2 in the MOFS-LE
group were similar to the findings of Wanten, et al. [10] who
reported that LEs containing MCT were associated with a higher
expression of leukocyte integrin b2.
Body weight did not differ significantly between
groups while it increased in both groups similarly before and after the
intervention. This observation is similar to that noted by Uthaya, et
al. [11]. However, others have reported better weight gain with S-LE
[3]. Length increments were not significantly different but was expected
to be better with MOFS-LE due to fish oil component of MOFS-LE, being
rich in docosahexaenoic acid [12].
The limitations of the study are that it was a
single-center study with a small sample size. Moreover, the evaluation
was done for a very short time period.
We conclude that MOFS lipid emulsions may result in
higher levels of soluble adhesion molecules, which could potentially
impact outcome in preterm septic neonates. Larger adequately powered
studies would be needed to study the clinical and functional
significance of these effects.
Contributors: MA: acquisition of clinical
data, drafting the manuscript. YW: analysis and interpretation of data,
revising the article draft critically for important intellectual
content. BS: conception and design of the study, analysis and
interpretation of the clinical data, and drafting the article. AM:
acquisition of laboratory data, analysis and interpretation of the
clinical data, and drafting the article. All author approved the final
version to be published and agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or integrity
of any part of the work are appropriately investigated and resolved.
Funding: None; Competing Interest: None
stated.
|
What This Study Adds?
•
Higher levels of soluble adhesion molecule leukocyte
integrin b2 are found in
preterm infants receiving Medium Chain
triglyceride-Olive-Fish-Soy (MOFS)-based lipid emulsion in
comparison to those receiving soybean-based lipid emulsion.
|
References
1. Ramel SE, Brown LD, Georgieff MK. The impact of
neonatal illness on nutritional requirements: one size does not fit all.
Curr Pediatr Rep. 2014;2:248-54.
2. Christmann V, Visser R, Engelkes M, Grauw AM,
Goudoever JB, Heijst AFJ. The enigma to achieve normal postnatal growth
in preterm infants-using parenteral or enteral nutrition? Acta Paediatr.
2013;102:471-9.
3. Kapoor V, Glover R, Malviya MN. Alternative lipid
emulsions versus pure soy oil based lipid emulsions for parenterally fed
preterm infants. Cochrane Database Syst Rev. 2015;12:CD009172.
4. Koletzko B, Goulet O. Fish oil containing
intravenous lipid emulsions in parenteral nutrition-associated
cholestatic liver disease. Curr Opin Clin Nutr Metab Care.
2010;13:321-6.
5. Koletzko B, Agostoni C, Carlson SE, Clandinin T,
Hornstra G, Neuringer M, et al. Long chain polyunsaturated fatty
acids (LC-PUFA) and perinatal development. Acta Paediatr. 2001;90:460-4.
6. Sala-Vila A, Barbosa VM, Calder PC. Olive oil in
parenteral nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:165-74.
7. Ley K, Laudanna C, Cybulsky MI, Nourshargh S.
Getting to the site of inflammation: The leukocyte adhesion cascade
updated. Nat Rev Immunol. 2007;7:678-9.
8. Edgar JDM, Gabriel V, Gallimore JR, McMillan SA,
Grant J. A prospective study of the sensitivity, specificity and
diagnostic performance of soluble intercellular adhesion molecule 1,
highly sensitive C-reactive protein, soluble E-selectin and serum
amyloid A in the diagnosis of neonatal infection. BMC Pediatr.
2010;10:22.
9. Briassoulis G, Papassotiriou I, Mavrikiou M,
Lazaropoulou C, Margeli A. Longitudinal course and clinical significance
of TGF- a1, sL-and
sE-Selectins and sICAM-1 levels during severe acute stress in children.
Clin Biochem. 2007;40:299-304.
10. Wanten GJA, Geijtenbeek TBH, Raymakers RAP, van
Kooyk Y, Roos D, Jansen JBMJ, et al. Medium-chain,
triglyceride-containing lipid emulsions increase human neutrophil beta2
integrin expression, adhesion, and degranulation. J Parenter Enter Nutr.
2000;24:228-33.
11. Uthaya SN, Liu X, Babalis D, Doré CJ, Warwick J,
Bell J, et al. Nutritional evaluation and optimisation in
neonates (NEON) trial of amino acid regimen and intravenous lipid
composition in preterm parenteral nutrition: A randomised double-blind
controlled trial. Effic Mech Eval. 2016;3.
12. López-Alarcón M, Bernabe-García M, del Valle O,
González-Moreno G, Martínez-Basilea A, Villegas R. Oral administration
of docosahexaenoic acid attenuates interleukin-1 b
response and clinical course of septic neonates. Nutrition.
2012;28:384-90.
|
|
|
 |
|

