lectroencephalography (EEG) is a non-invasive,
readily available and inexpensive investigation to study the neuronal
dysfunction and abnormal cortical excitability in children who present
with seizures [1]. Traditional analog EEG machines are being replaced by
digital EEG with simultaneous video recording. Surface scalp EEG
recording can be conventional short-term recording (30 minutes) or
long-term video EEG record (for witnessing and localizing seizure
activity).
Sensitivity and specificity of surface scalp EEG to
localize the epileptogenic focus depends on factors such as age, type of
epilepsy, and nature of EEG recording [2]. Ictal EEG (EEG recording
during the seizure) helps to recognize the type of seizure that may not
be evident from history and for localizing the epileptogenic zone [3].
Electrocorticography (ECoG) or intracranial EEG is useful for invasive
recording of cortical electrical activity by use of electrodes directly
on the surface of brain (subdural grids or strips) or deep inside the
brain (depth electrodes) [4]. It helps to localize the epileptogenic
zone and to map cortical functional areas in drug-resistant epilepsy.
Detailed discussion of invasive EEG studies and its role in planning
epilepsy surgery is beyond the scope of this review. American Clinical
Neurophysiology Society (ACNS) has published technical guidelines for
recording digital EEG [5].
Principle of EEG
EEG measures the electropotential difference that
arises from the ion trafficking between two points on the scalp.
Potential differences between electrodes are amplified and the net
signal from each amplifier is displayed on a monitor to provide a
graphic record. EEG signals are generated by the summation of excitatory
and inhibitory post-synaptic potentials from large, vertically-oriented
pyramidal neurons located in layer III, V and VI [6]. These EEG signals
are synchronized by subcortical structures like thalamus and brainstem
reticular formation. Sleep spindles are considered a result of these
thalamocortical phenomena [6]. Large numbers of cortical spikes are not
recordable on a routine scalp EEG due to attenuating effect of
cerebrospinal fluid, duramater and skull scalp tissue.
Technical Aspects
The surface EEG electrodes made of gold or silver
discs (silver chloride) are placed at standard points over scalp with a
conductive paste. The International 10-20 system (10 and 20% gap between
electrodes) is used for electrode placement [7]. Pediatric EEG routinely
requires the placement of 21 electrodes on the scalp with fewer
electrodes (minimum of 12 electrodes) in neonates and young infants [8].
EEG can be performed in a laboratory or bedside ambulatory EEG could be
used. Additional channels of electrocardiogram (EKG) and respiration are
recommended to record physiologic artefacts. EKG during EEG recording
helps detect ictal arrhythmia and asystole in children with epilepsy who
are prone to sudden unexpected death (SUDEP) [9]. Surface
electromyography (EMG) during EEG helps to distinguish epileptic from
non-epileptic movements [10]. Electrodes are named according to the
underlying area of brain: FP: frontopolar, F: frontal, P: parietal, T:
temporal, and O: occipital. Central electrodes are abbreviated as Z [Fz,
Cz, Pz] and referential electrodes include post auricular (A1, A2). The
odd numbers (Fp1, F3, P3, C3, T3, T5, T7, O1) depict left side of the
hemisphere and even numbers (Fp2, F4, C4, P4, T4, T6, O2) for the right
side. These electrodes are either fixed to scalp using conductive paste
or electrodes fixed onto a head cap are used.
Patient Preparation
The scalp should be clean and dry. Patient should be
instructed to consume antiepileptic drugs (AED) as prescribed. However,
AED doses can be reduced or discontinued to facilitate seizure
occurrence during long-term video EEG monitoring [11]. Children can have
their routine breakfast on the day of appointment. Routine EEG
recordings usually lasts for 30 minutes, including hyperventilation for
3 minute and intermittent photic stimulation at 1-30 Hz [5]. Long-term
video EEG recordings are particularly useful for pre-surgical evaluation
for epilepsy surgery [12]. An ideal EEG should include both awake and
sleep record. However, sleep EEG is preferred in younger children
considering excessive movement artefacts during the wakeful state.
Moreover, sleep EEG provides vital information on maturation of brain
[8]. Sleep deprived EEG protocol requires 4-6 hours of sleep deprivation
[13]. Children older than 3 years could be kept awake until midnight and
woken up at 5:00 AM on the morning of the test. Sleep deprivation is
considered to enhance sensitivity of EEG [14]. Triclofos (20
mg/kg/dose), melatonin (2-6 mg/dose) or clonidine (0.05-0.2 mg) can be
used for sedation [15]. Intravenous midazolam should not be used to
induce sleep due to its suppressive effect on epileptiform discharges.
In addition to sleep deprivation, yield of EEG can be increased by
repeat recording, prolonging the duration of recording, increasing the
number of channels during procedure, simultaneous video recording, and
recording both awake and sleep state [16].
Activation Procedure
Infants, young children and children with suspected
focal epilepsies require sleep EEG record [17-19]. Sleep EEG is
essential for diagnosis of epileptic encephalopathy and continuous spike
waves during slow sleep (CSWS) [20]. EEG in awake state is useful to
detect generalized epilepsies. Activation procedure include
hyperventilation (3 Hz spike wave pattern in absence epilepsies) and
intermittent photic stimulation (4-6 Hz generalized epileptiform
discharges in Juvenile myoclonic epilepsy). Other activation procedures
indicated for specific conditions are: fixation of sensitivity (late
onset occipital lobe epilepsy), precipitation by trigger (e.g.,
video watching) in reflex epilepsies, and suggestion to precipitate
paroxysmal non-epileptic events [21]. Reactivity of EEG background is
observed by asking the child to close and open the eyes or by touching
his various body parts.
EEG Requisition
An EEG requisition form from clinician must contain
basic demographic profile (name, age, gender, telephone number or
email), type of seizure, frequency of seizure, age at onset, indication
of EEG, neuroimaging findings, any previous EEG findings, and name of
antiepileptic drugs. Neurophysician can decide on the EEG protocol based
on clinical diagnosis. For example, in a child with suspected absence
epilepsy, one would prefer an awake EEG record with hyperventilation.
Sleep deprived sleep EEG record will be considered in a child with
suspected focal epilepsy.
eeg interpretation
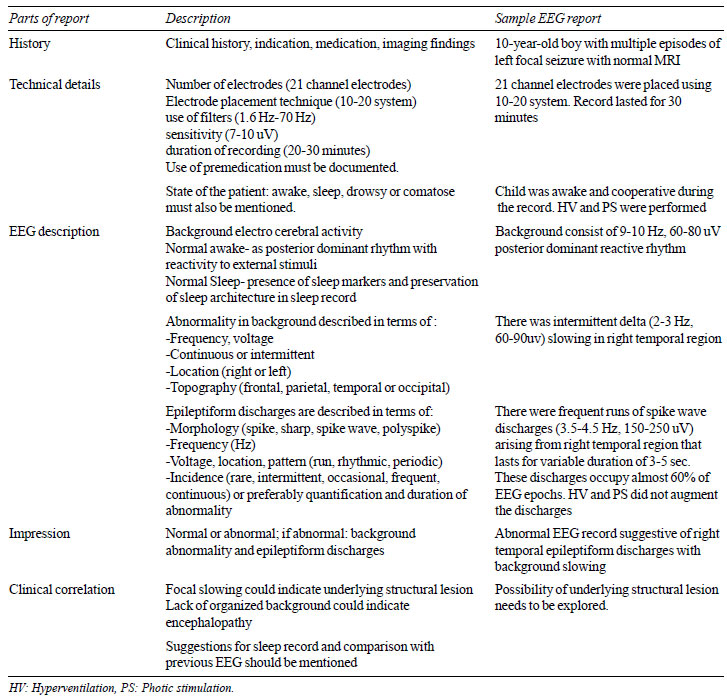
ACNS guidelines have outlined five essential
components of an EEG report (Table I) [22]. Abnormalities
in EEG can be divided into background abnormalities and abnormal
epileptiform discharges. Background gives information about the
neurologic state of the child. Normal awake record consists of posterior
dominant alpha rhythm (8-13 Hz) with reactivity to eye closure.
Similarly, sleep background consist of sleep markers of non-REM sleep
such as sleep spindles, vertex waves, and K-complexes (Web Fig.
1). Epileptiform discharges have distinct waveforms classified as
spikes (<70 ms) or sharp wave (70-200 ms). EEG findings in common
self-limited epilepsies and epileptic encephalopathy in children have
been summarized in Table II and Table III,
respectively.
|
TABLE I Essential Component of an Eeg
Report (as per Acns Guidelines for Reporting Eeg).
|
 |
|
TABLE II Clinical and EEG Findings in
Self-limited Epileptic Seizures and Syndromes
|
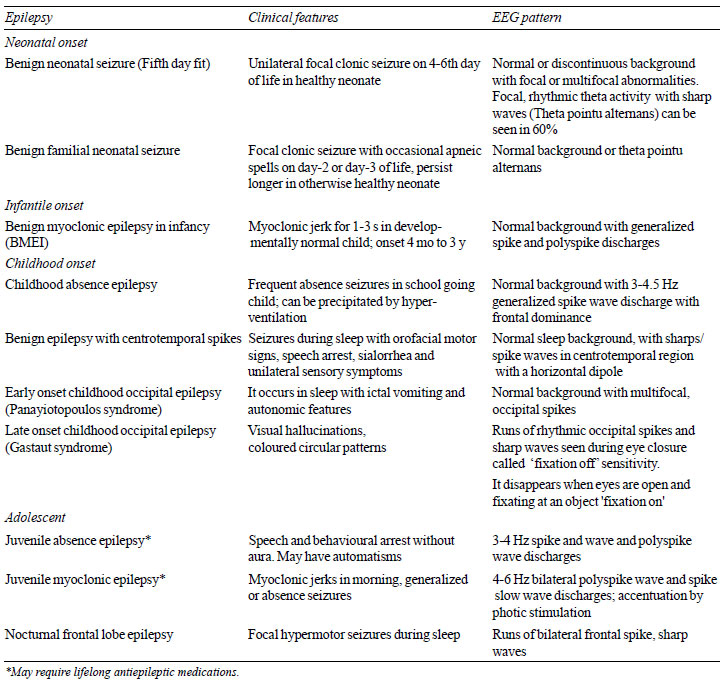 |
|
TABLE III Clinical and Eeg Features of
Epileptic Encephalopathy
|
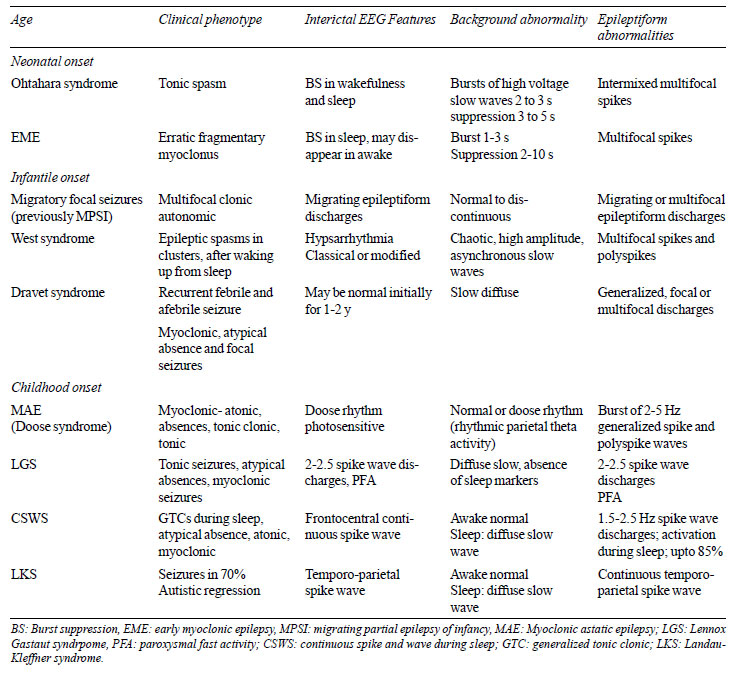 |
Pitfalls of EEG
Surface EEG can be normal in few epileptic conditions
in children, especially those with remote and deep location of
epileptogenic lesion such as interhemispheric area, and mesial and basal
cortex [19]. Few genetic types of epilepsy such as benign familial
neonatal epilepsy and benign familial infantile epilepsy can have normal
interictal EEG. Epileptiform discharges are found in 0-5.6% of normal
healthy children and 0.5% of adults without any event of seizure [23].
EEG can be abnormal in approximately 5.7-59% of children with autism
spectrum disorder without any clinical seizures [24]. Photic driving
response can routinely be found in patients with migraine without any
epilepsy [25]. EEG is often reported by neurologist, pediatric
neurologist, psychiatrist, neurophysiologist and other physicians with
interest in EEG. Hence, there is lot of variability and subjectivity in
reporting pediatric EEG. Exclusive training and experience to interpret
pediatric EEGs is essential to understand the normal age-dependent
variations and correct characterization of epileptiform discharges. Some
of the common errors in pediatric EEG reporting include misinterpreting
movement artefacts, high amplitude delta slowing during hyperventilation
and normal sleep markers including vertex waves, and K complexes as
epileptiform discharges (Web Fig. 1). Other benign
epileptiform variants like wicket waves, benign epileptiform transients
of sleep (BETS) and rhythmic midtemporal theta bursts of drowsiness
(RMTD) can mimic epileptiform discharges to a naïve reader [26]. Awake
EEG record can be normal in children with suspected rolandic epilepsy,
structural focal epilepsy or CSWS. These abnormalities are detected only
on sleep EEG record. Similarly, among those with suspected childhood
absence epilepsy and juvenile myoclonic epilepsy, hyperventilation and
photic stimulation during awake EEG record is mandatory.
Indications of EEG
EEG is an adjunct to clinical evaluation and should
be interpreted in clinical context. Indications of using and not using
EEG are summarized in (Box 1). Diagnosis of epilepsy
should not be reached solely on the basis of EEG findings [27]. A wrong
diagnosis of epilepsy has widespread social implications apart from side
effects of antiepileptic drugs and restriction of physical activities.
EEG is often misused in evaluation of a child with abnormal paroxysm to
differentiate epileptic from non-epileptic event [28].
Over-interpretation of EEG abnormalities, including focal slowing,
generalized and focal epileptiform discharges has often led to syncope
being misdiagnosed as epileptic seizures [21]. There is limited role of
EEG in children with breath holding spells. Common reasons for
misinterpretation of EEG include poor expertise, lack of good quality
recording, inappropriate indication, and absence of clinical correlation
[27]. Routine surface scalp EEG report should ideally comprise five
components: history, technical description, EEG description, impression
and clinical correlation [22].
|
Box 1 Indications of
Electroencephalography in Pediatric Epilepsy
|
|
When to use
• EEG helps in differentiating epileptic from
non-epileptic clinical event. Video EEG with capture of ictal
event is useful adjunct to support clinical possibility of
epileptic event.
• To classify the type of epilepsy into focal
or generalized epilepsy and diagnosis of various
electro-clinical epilepsy syndrome.
• Video EEG monitoring with spell capture is
vital to localize the epileptic focus in case of focal epilepsy.
• To characterize the type of epileptic
syndrome based on cluster of clinical seizure semiology, age at
onset and EEG findings.
• It helps clinician decide on tapering
antiepileptic drugs after a seizure free interval and to predict
possible relapse after tapering antiepileptic drug.
• To guide about the etiology in a case of
meningo-encephalitis (e.g., periodic lateralized
epileptiform discharges in case of herpes simplex encephalitis).
• To diagnose NCSE in case of prolonged coma
after status epilepticus or encephalopathy of unknown etiology.
• In children with cognitive or language
regression even without seizures, it is indispensable to rule
out epileptic encephalopathy like CSWS and LKS.
• To prognosticate an epileptic disorder
e.g., Periodic complexes, triphasic waves in a sick patient
in ICU is suggestive of poor prognosis. Also, presence of
epileptiform discharges predicts seizure recurrence in epilepsy.
• Ancillary test for documentation of brain
death.
When not to use
• To exclude a diagnosis of epilepsy; since
epilepsy is largely a clinical diagnosis.
• To monitor the progress of epilepsy with
EEG.
(Note: In a children with epilepsy, new onset
clinical features like cognitive decline or behavioural issue
warrants fresh EEG to rule out NCSE).
• To monitor the efficacy of antiepileptic
drugs (AED) in epilepsy except in infantile spasm, LKS, CSWS or
absence epilepsy where there could be no change with AED.
(Note: Valproate and benzodiazepines can
decrease the spike burden).
• Intracranial space occupying lesions
including stroke without any history of seizures or raised
intracranial pressure to form the basis of starting prophylactic
AED.
• Clinical history that clearly suggests
paroxysmal non epileptic event like shuddering spells,
gratification, and syncopal attacks.
CSWS: Continuous spike wave in sleep, LKS: Landau Kleffner
syndrome, NCSE: Non convulsive status epilepticus, AED:
antiepileptic drug, EEG: Electroencephalography.
|
First Unprovoked Seizure
First unprovoked seizure (FUS) is defined as first
non-febrile seizure that cannot be explained by an immediate, obvious
precipitating cause such as head trauma or intracranial infection. In
developing countries including India, focal lesions such as
neurocysticercosis (NCC) and tuberculoma are common causes of first
unprovoked seizure in children [29]. Thus, in many centers, neuroimaging
often precedes EEG in evaluation of such children. EEG is recommended as
first tier investigation among children with first unprovoked seizure
for diagnosis of seizure, epilepsy type and an epileptic syndrome. It
may be useful for prediction of long-term outcome or recurrence [30].
Children who have focal epileptiform discharges on EEG have a higher
risk for recurrence when compared to those with normal EEG [31].
However, in obvious etiology like neuro-cysticercosis or tuberculoma,
EEG should not be routinely requested at outset. EEG may be helpful
before withdrawing AED in such patients. Among children with new onset
seizures, 18-56% display epileptiform discharges on initial EEG and 15%
will never show abnormal findings [32]. EEG done early within first 24
hours of seizure shows background and epileptiform abnormality more
frequently [33]. These background abnormalities are often transient and
warrant repeat EEG after certain duration to look for persistence of
abnormality.
Characterization of Type of Seizure and Syndromic
Diagnosis
EEG abnormalities are broadly divided into background
abnormalities and abnormal epileptiform waveforms. Background
abnormalities include diffuse slowing, asymmetric slowing, discontinuous
background and electrodecremental response. Group of disorders with
discontinuous EEG background where epileptiform activities contribute to
encephalopathy or non-attainment of milestones is called epileptic
encephalopathy. This includes Early myoclonic encephalopathy, Ohtahara
syndrome, West syndrome (Web Fig. 2), Lennox Gestaut
syndrome, and Landau Kleffner syndrome. There are signature EEG features
to diagnose epileptic encephalopathies as these conditions have urgent
treatment implications (Table III). Interictal EEG can be
categorized into focal or generalized based on the morphology of
epileptiform discharges and organization of background activity.
Generalized spike and spike-wave discharges with normal interictal
background activity is observed in childhood/juvenile absence epilepsy
(CAE/JAE), epilepsy with myoclonic astatic seizures (Doose syndrome),
juvenile myoclonic epilepsy (JME) (Web Fig. 3), and
epilepsy with eyelid myoclonia (Jeavon syndrome). There are a group of
self-limited epilepsies with focal stereotyped spikes wherein the focal
spikes can be seen with normal interictal EEG background. This includes
Rolandic epilepsy (Web Fig. 4), Panayiotopoulos syndrome
and benign occipital epilepsies. Children with structural lesion like
Neurocysticercosis, glioma or vascular lesion can also have focal
epileptiform discharges (Web Fig. 5). Children with
subacute sclerosing panencephalitis can have periodic epileptiform
discharges (Web Fig. 6).
Status Epilepticus
EEG is required to rule out nonconvulsive status
epilepticus among those with no improvement of altered sensorium
following convulsive seizures [34]. EEG is also useful adjunct to
monitor seizure activity among those with neuromuscular blockade (which
might mask convulsive activity) and high dose suppressive therapy for
refractory status epilepticus. Among those with refractory status
epilepticus, suppression of epileptiform discharges to achieve burst
suppression on EEG is often considered end point for titrating the dose
of antiepileptic and anesthetic agents [35].
Comatose Child
Continuous EEG monitoring in intensive care unit
(ICU) setting is ideal during management of a child with refractory
status epilepticus, heavy sedation, those on neuromuscular blocker, or
those being treated with barbiturate for raised intracranial pressure
[36]. A comatose child with past history of seizure or seizure like
activity requires an EEG to rule out non convulsive status epilepticus.
The most common EEG finding in a child with coma is diffuse slowing with
reduction in amplitude of waveform. Triphasic waves on EEG could point
to ward underlying metabolic disorder. Similarly, periodic lateralized
epileptiform discharges (PLED) suggest a focus of irritable cortex seen
in space occupying lesion or herpes encephalitis. Serial EEG can help
with prognostication. In children with post anoxic coma, burst
suppression or isoelectric pattern on EEG is a poor prognostic marker
for recovery [37]. EEG monitoring in ICU setting has limited role
because of environmental noise and use of sedative drugs.
Febrile Seizure
There is no role of EEG in children with simple
febrile seizures [38]. However, EEG is more likely to be abnormal among
those with complex febrile seizures, including febrile status
epilepticus and focal febrile seizures. Focal epileptiform abnormalities
were significantly more frequent among children with complex febrile
seizure who subsequently developed epilepsy [39]. However, epileptiform
EEG has poor positive predictive value for subsequent development of
epilepsy [40], and there is no evidence to support or refute the use of
EEG after complex febrile seizure [41]. Hence, there is limited utility
of EEG among children with febrile seizures with lack of clinical
significance of an abnormal EEG in predicting recurrence or subsequent
development of epilepsy.
Discontinuation of AED
Majority of children who are seizure-free for a
duration of at least 2 years or more have minimal risk for seizure
recurrence [42]. However, the risk of recurrence following withdrawal
will depend on type of epilepsy, polytherapy, abnormality on
neuroimaging and EEG [43]. Patients with intellectual disability,
abnormal neurological examination, older age at onset of seizure, focal
seizures and epileptiform abnormalities on EEG have a higher risk of
relapse [42]. Abnormal EEG at the time of AED withdrawal has been shown
to be associated with a relative risk of recurrence of 1.45 (95% CI
1.18-1.79) [44]. There is an emerging interest on serial EEG monitoring
during AED withdrawal to predict risk of recurrence. In a study on 84
children who had seizure recurrence despite normal EEG at the time of
drug withdrawal, 24 had abnormal EEG during AED withdrawal [45].
Conclusion
A normal interictal EEG does not exclude the
diagnosis of epilepsy. EEG is useful to establish the diagnosis of
epilepsy, classify the type of epilepsy and to rule out nonconvulsive
status epilepticus among children with unexplained coma. EEG is often
misused to justify the need for AED among children with clear history of
paroxysmal non-epileptic events, headache, simple febrile seizures and
head trauma. An abnormal EEG report should always be interpreted in
clinical context.
1. Clancy RR, Bergvist AGC, Dlugos DJ, Nordli DR.
Normal pediatric EEG: neonate and children. In: Ebersole JS, editors.
Current Practice of Clinical Electro-encephalography. 4th ed.
Philadelphia: Lippincott Williams & Wilkins; 2014. p. 125-212.
2. Noachtar S, Peters AS. Semiology of epileptic
seizures: A critical review. Epilepsy Behav. 2009;15:2-9.
3. Yoshinaga H, Hattori J, Ohta H, Asano T, Ogino T,
Kobayashi K, et al. Utility of the scalp-recorded ictal EEG in
childhood epilepsy. Epilepsia. 2001;42:772-7.
4. Fernández IS, Loddenkemper T. Electrocorticography
for seizure foci mapping in epilepsy surgery. J Clin Neurophysiol.
2013;30:554-70.
5. Halford JJ, Sabau D, Drislane FW, Tsuchida TN,
Sinha SR. American Clinical Neurophysiology Society Guideline 4:
Recording Clinical EEG on Digital Media. J Clin Neurophysiol.
2016;33:317-9.
6. Olejniczak P. Neurophysiologic basis of EEG. J
Clin Neurophysiol. 2006;23:186-9.
7. Maus D, Epstein CM, Herman ST. Digital EEG. In:
Schomer DL, Lopes Da Silva FH, (editors). Niedermeyer's
Electroencephalography: Basic Principles, Clinical Applications, and
Related Fields. 5th ed. Philadelphia: Lippincott Williams & Wilkins;.
2005. p. 127-38.
8. Kaminska A, Cheliout-Heraut F, Eisermann M,
Touzery de Villepin A, Lamblin MD. EEG in children, in the laboratory or
at the patient's bedside. Neurophysiol Clin Clin Neurophysiol.
2015;45:65-74.
9. Bartlam R, Mohanraj R. Ictal bradyarrhythmias and
asystole requiring pacemaker implantation: Combined EEG-ECG analysis of
5 cases. Epilepsy Behav. 2016;64:212-5.
10. Hill AT, Briggs BA, Seneviratne U. Simultaneous
recording of EEG and electromyographic polygraphy increases the
diagnostic yield of video-EEG monitoring. J Clin Neurophysiol.
2014;31:203-7.
11. Rizvi SAA, Hernandez-Ronquillo L, Wu A, Téllez
Zenteno JF. Is rapid withdrawal of anti-epileptic drug therapy during
video EEG monitoring safe and efficacious? Epilepsy Res.
2014;108:755-64.
12. Kobulashvili T, Höfler J, Dobesberger J, Ernst F,
Ryvlin P, Cross JH, et al. Current practices in long-term
video-EEG monitoring services: A survey among partners of the epilepsy
pilot network of reference for refractory epilepsy and epilepsy surgery.
Seizure. 2016;38:38-45.
13. DeRoos ST, Chillag KL, Keeler M, Gilbert DL.
Effects of sleep deprivation on the pediatric electroencephalogram.
Pediatrics. 2009;123:703-8.
14. Giorgi FS, Guida M, Caciagli L, Maestri M,
Carnicelli L, Bonanni E, et al. What is the role for EEG after
sleep deprivation in the diagnosis of epilepsy? Issues, controversies,
and future directions. Neurosci Biobehav Rev. 2014;47:533-48.
15. Jain P, Sharma S, Sharma A, Goel S, Jose A, Aneja
S. Efficacy and safety of oral triclofos as sedative for children
undergoing sleep electroencephalogram: An observational study. J Pediatr
Neurosci. 2016;11:105-8.
16. Panayiotopoulos CP. EEG and Brain imaging. In:
Panayiotopoulos CP, editors. A clincal guide to epileptic syndromes and
their treatment. 2nd ed. London: Springer publication; 2010. p. 147-71.
17. Pillai J, Sperling MR. Interictal EEG and the
diagnosis of epilepsy. Epilepsia. 2006;47:14-22.
18. Noachtar S, Rémi J. The role of EEG in epilepsy:
A critical review. Epilepsy Behav. 2009;15:22-33.
19. Fowle AJ, Binnie CD. Uses and abuses of the EEG
in epilepsy. Epilepsia. 2000;41:S10-18.
20. Gencpinar P, Dundar NO, Tekgul H. Electrical
status epilepticus in sleep (ESES)/continuous spikes and waves during
slow sleep (CSWS) syndrome in children: An electroclinical evaluation
according to the EEG patterns. Epilepsy Behav. 2016;61:107-11.
21. Fattouch J, Casciato S, Lapenta L, Morano A,
Fanella M, Lombardi L, et al. The spectrum of epileptic syndromes
with fixation off sensitivity persisting in adult life. Epilepsia.
2013;54:59-65.
22. Tatum WO, Olga S, Ochoa JG, Munger Clary H, Cheek
J, Drislane F, et al. American Clinical Neurophysiology Society
Guideline 7: Guidelines for EEG Reporting. J Clin Neurophysiol.
2016;33:328-32.
23. So EL. Interictal epileptiform discharges in
persons without a history of seizures: What do they mean? J Clin
Neurophysiol. 2010;27:229-38.
24. Spence SJ, Schneider MT. The role of epilepsy and
epileptiform EEGs in autism spectrum disorders. Pediatr Res.
2009;65:599-606.
25. Fogang Y, Gérard P, De Pasqua V, Pepin JL, Ndiaye
M, Magis D, et al. Analysis and clinical correlates of 20 Hz
photic driving on routine EEG in migraine. Acta Neurol Belg.
2015;115:39-45.
26. Hernandez-Frau PE, Benbadis SR. Pearls and
oysters: errors in EEG interpretations: What is misinterpreted besides
temporal sharp transients? Neurology. 2011;76: 57-9.
27. Benbadis SR. The tragedy of over-read EEGs and
wrong diagnoses of epilepsy. Expert Rev Neurother. 2010;10:343.
28. Laffan A, Langan Y. Understanding EEG indications
and reports: A survey of NCHDs. Ir Med J. 2013;106:59-60.
29. Sahu PS, Seepana J, Padela S, Sahu AK,
Subbarayudu S, Barua A. Neurocysticercosis in children presenting with
afebrile seizure: clinical profile, imaging and serodiagnosis. Rev Inst
Med Trop São Paulo. 2014;56: 253-8.
30. Pohlmann-Eden B, Newton M. First seizure: EEG and
neuroimaging following an epileptic seizure. Epilepsia.
2008;49(Suppl.1):19-25.
31. Mizorogi S, Kanemura H, Sano F, Sugita K, Aihara
M. Risk factors for seizure recurrence in children after first
unprovoked seizure. Pediatr Int. 2015;57:665-9.
32. Wirrell EC. Prognostic significance of interictal
epileptiform discharges in newly diagnosed seizure disorders. J Clin
Neurophysiol. 2010;27:239-48.
33. King MA, Newton MR, Jackson GD, Fitt GJ, Mitchell
LA, Silvapulle MJ, et al. Epileptology of the first-seizure
presentation: A clinical, electroencephalographic, and magnetic
resonance imaging study of 300 consecutive patients. Lancet.
1998;352:1007-11.
34. Trinka E, Leitinger M. Which EEG patterns in coma
are nonconvulsive status epilepticus? Epilepsy Behav. 2015;49:203-22.
35. Bergey GK. Refractory Status Epilepticus: Is EEG
burst suppression an appropriate treatment target during drug-induced
coma? What is the holy grail? Epilepsy Curr. 2006;6:119-20.
36. Seshia SS, Bingham WT, Kirkham FJ, Sadanand V.
Nontraumatic coma in children and adolescents: Diagnosis and management.
Neurol Clin. 2011;29:1007-43.
37. Zandbergen EG, de Haan RJ, Stoutenbeek CP,
Koelman JH, Hijdra A. Systematic review of early prediction of poor
outcome in anoxic-ischaemic coma. Lancet. 1998; 352:1808-12.
38. Subcommittee on Febrile Seizures, American
Academy of Pediatrics. Neurodiagnostic evaluation of the child with a
simple febrile seizure. Pediatrics. 2011;127:389-94.
39. Kim H, Byun SH, Kim JS, Lim BC, Chae J-H, Choi J,
et al. Clinical and EEG risk factors for subsequent epilepsy in
patients with complex febrile seizures. Epilepsy Res. 2013;105:158-63.
40. Harini C, Nagarajan E, Kimia AA, de Carvalho RM,
An S, Bergin AM, et al. Utility of initial EEG in first complex
febrile seizure. Epilepsy Behav. 2015;52:200-4.
41. Shah PB, James S, Elayaraja S. EEG for children
with complex febrile seizures. Cochrane Database Syst Rev.
2015;CD009196.
42. Beghi E, Giussani G, Grosso S, Iudice A, La Neve
A, Pisani F, et al. Withdrawal of antiepileptic drugs: guidelines
of the Italian League Against Epilepsy. Epilepsia. 2013 ;54:2-12.
43. Afshari D, Moradian N. Evaluating the rate of
recurrence of epilepsy after therapy discontinuation in 2-year
seizure-free epileptic patients. Int J Neurosci. 2012;122:598-601.
44. Berg AT, Shinnar S. Relapse following
discontinuation of antiepileptic drugs: A meta-analysis. Neurology.
1994;44:601-8.
45. Verrotti A, Matricardi S, Di Giacomo DL, Rapino D, Chiarelli F,
Coppola G. Neuropsychological impairment in children with Rolandic
epilepsy and in their siblings. Epilepsy Behav. 2013;28:108-12.

