|
|
|
Indian Pediatr 2016;53: 920-922 |
 |
Central or Peripheral
Precocious Puberty: Diagnostic Difficulties
|
|
* María Eugenia López Valverde,
#Ana Villamañán
Montero, #Aránzazu
Garza Espí and
#Antonio de
Arriba Muñoz
From Departments of *Endocrinology and Nutrition, and
#Pediatric Endocrinology; Hospital Universitario Miguel
Servet, Spain.
Correspondence to: Dr María Eugenia López Valverde.
Endocrinology and Nutrition, Hospital Universitario Miguel Servet,
Spain. [email protected]
Received: August 24, 2015;
Initial review: October 20, 2015;
Accepted: May 27, 2016.
|
Background: An underlying identifiable organic
cause is present in up to 50% cases of central precocious puberty in
male patients. Case characteristics: A 7-years-8-months-old
presented with delayed puberal development. Analytical examinations
showed suppressed basal and stimulated levels of testosterone, LH and
FSH. Abdominal ultrasound and contrast cranial magnetic resonance
results were initially negative. Outcome: Germinoma was found on
cranial computer tomography. Conclusion: There is often a wide
time-lapse between symptoms and diagnosis of germinoma, so frequent
monitoring is vital.
Keywords: Central nervous system tumor, Germinoma,
Hypopituitarism.
|
|
Central precocious puberty is
of idiopathic origin in upto 95% of girls, while in up to 50% of males
there is an underlying identifiable organic cause [1]. It is therefore
important to take into account that diagnosis of idiopathic central
precocious puberty in boys must be a diagnosis of exclusion. One of
several organic causes is germinoma, an unusual tumour with variable
clinical manifestations. A specific characteristic of these tumours is
the considerable length of time that elapses between their onset and
their correct diagnosis on MRI scans.
Case-report
A male patient aged 7
years 8 months presented with a 3-month history of facial
acne and increase in testicular volume. Upon examination, he had a
testicular volume of 6 ml, penis stage IV, pubarche stage I; weight 25
kg (-0.46 SDS), height 124 cm, (-0.62 SDS), body mass index 16.26 kg/m2
and considerable facial acne, and a bone age of 9
years 3 months (predicted adult height 168.9 cm).
The remaining systemic examination showed normal results and the patient
did not have any neurological symptoms.
The results of the biochemical analyses showed
suppressed levels of LH and FSH (0.11 mUI/mL), androstenedione 0.09 ng/mL
(normal 0.3-3.1), DHEA-S 17 mcg/dL (normal 24±22), total testosterone
1.96 ng/mL (normal 1.75-7.81) and free testosterone 2.31 pg/mL (normal
0.04-0.09). In LHRH test, It was administered one single injection of
LHRH (100 µg intravenous), with basal blood extraction, and 20, 30 and
60 minutes afterwards: LH 0.11-0.71 mUI/mL and FSH 0.11-0.2 mUI/mL.
Serum b-HCG
levels were 5 mUI/mL (normal <1.2) and
afetoprotein 1.56 ng/mL
(normal 0-15).
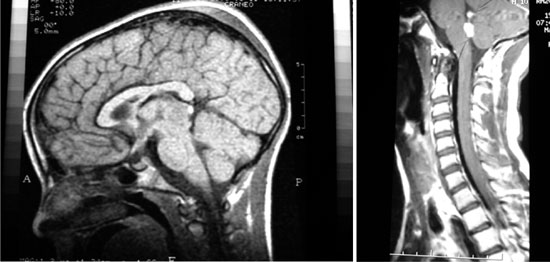 |
| (a) |
(b) |
|
Fig. 1 Germinoma MRI imaging. (a)
MRI brain sagittal section showing germinoma in pineal region
(arrow). (b) Hyperintense mass lesions suggestive of germinoma
metastases (arrow) in fourth ventricle.
|
A gadolinium-enhanced cranial magnetic resonance
imaging (MRI) and abdominal ultrasound scan showed no abnormalities, but
following a testicular scan, a 1 cm nodule in the left testicle was
detected. Therefore, peripheral precocious puberty was suspected, and a
testicular biopsy was taken. However, given that the results of the
biopsy were normal, this then indicated a likely case of testotoxicosis.
Treatment with Keto-conazole was prescribed, and following three months
of treatment, his signs of puberty effectively stabilized (testicles 5-6
mL, improvement in facial acne), height 130.4 cm (0.28 SDS) and weight
(-0.3 SDS). However, due to a subsequent onset of polyuria and
polydipsia, a computerized tomography scan (CT) and contrast-enhanced
cranial MRI scan were taken, both of which presented normal results.
Additionally, a blood test was taken, which confirmed that he was
suffering from diabetes insipidus. The patient required desmopressin
treatment, which controlled the symptomatology. It was thought
b-HCG levels
were normal, so they were not monitored and repeat CT and MRI, were
again normal.
Around 21 months later, following continuous clinical
monitoring, the patient began with complaints of headaches and seizures.
Another contrast Gadolinium enhanced MRI scan detected a 2.2×2 cm pineal
tumour with triventricular hydrocephalus, diagnosed as an hCG secreting
tumour (high a-fetoprotein
and bhCG in
lumbar cerebrospinal fluid and on a ventricular level), which required
radiotherapy and chemotherapy. Following this treatment, the tumour was
no longer visible on the MRI scan, and tumour markers were negative. At
the age of 9 years 9 months,
the patient showed a regression in pubertal development (testicular
volume 3-4 mL, penis stage IV, no signs of pubarche), with a slow growth
rate of 0.3 cm/year, a height of 134.1cm (-1.38 SDS), weight 26.5 kg
(-1.07 SDS), and a bone age of 12 years. The clonidine stimulation test
confirmed a deficit of GH (maximum GH level: 0.38 ng/mL); however,
treatment was not given after a tumour recurrence was detected on the
MRI scan 18 months following diagnosis. The patient was administered
further radio- and chemo-therapy, but subsequently developed secondary
adrenal insufficiency which was treated with hydrocortisone. By the age
of 15 years 5 months, the
patient’s height was 145.3 cm (-3.65 SDS), weight 40.6 kg (-2.02 SDS),
testicular volume 10 mL, penis stage IV and pubarche stage IV, but he
had no facial or underarm hair. Basal LH and FSH levels were 1.8 and
13.4 mUI/mL respectively, and total testosterone levels were reduced
(0.07 ng/mL); GnRH stimulation test could not be done because health
state of the patient was seriously affected. So, treatment with
intramuscular testosterone was initiated for presence of secondary
hypogonadism.
The patient currently continues treatment of chronic
replacement therapy with hydrocortisone and testosterone.
Discussion
The diagnostic approach in precocious puberty can be
particularly complex. All of the information available initially
indicated peripheral precocious puberty, due to the absence of
gonadotropins, the negative results of the brain scan and initial
normality of the rest of the pituitary axis. However, there were certain
symptoms that implied central precocious puberty. Up to 60% of
germinomas do not show high âHCG levels, and where present, they tend to
be low, with averages values of 7.7 mUI/mL in some studies [2].
Additionally, diabetes insipidus is a symptom that frequently occurs in
up to 100% of patients with germinomas. Despite this, in this case,
there was no evidence on the serial cranial CT or MRI scans suggesting
any type of tumoral pathology. Thus, the most plausible suspected
diagnosis was peripheral precocious puberty, and the only viable option
was close patient monitoring.
CT and MRI scans are sensitive enough to diagnose
suprasellar or pituitary tumours. Neuroimaging was normal initially and
it was 2 years after the symptoms began that the pineal germinoma became
evident on the MRI brain scan. This is particularly relevant, because
there are few cases reported of germinoma in which symptoms actually
precede radiographic evidence [3]. Therefore, in the event that any
question should arise in diagnosis, it would seem advisable to monitor
b-HCG
levels rather than depending exclusively on the use of neuroimaging.
Although late diagnosis does not appear to affect survival rates in the
short-term [4], it does still have an impact on patient morbidity
because it increases the risk of disseminated disease and therefore
requires a more aggressive therapeutic treatment.
Given that germinoma is a radiosensitive tumour,
chemotherapy followed by irradiation enables a smaller dose of RT to be
given [5-7], which helps to achieve good long-term survival rates and
results in a decrease in side effects. We used a combination of both
treatments.
Due to the location of the tumour, the patient
developed hydrocephalus with secondary intracranial hypertension. When
this occurs, surgery is advised, but there is insufficient evidence to
determine whether a ventriculoperitoneal shunt or an endoscopic
ventriculo-stomy [8] is more suitable.
GH-deficiency is the earliest and most frequent
complication in patients who have been treated with cranial
radiotherapy. In this case, it was not treated due to the complexity of
the tumour and its subsequent recurrence. Additionally, both
radiotherapy and chemotherapy can cause hypogonadotropic hypogona-dism
in a considerable percentage of patients [9].
Diagnosis of germinomas is a complex issue.
Unfortunately, there is a small percentage of cases in which central
nervous system imaging do not reveal any pathological evidence.
Consequently, it is advisable to perform an exhaustive and frequent
follow-up of the patient, not only analytical or clinically, but also
with consecutive radiological brain imaging; this approach will prevent
from diagnostic delays and will minimize morbidity and mortality.
Contributors: All the authors have participated
in all the aspects of preparation of the manuscript.
Funding: None; Competing interest: None
stated.
References
1. Faizah MZ, Zuhanis AH, Rahmah R, Raja AA, Wu LL,
Dayang AA, et al. Precocious puberty in children: A review of
imaging findings. Biomed Imaging Interv J. 2012;8:e6.
2. Allen J, Chacko J, Donahue B, Dhall G, Kretschmar
C, Jakacki R, et al. Diagnostic sensitivity of serum and lumbar
CSF bHCG in newly Diagnosed CNS Germinoma. Pediatr Blood Cancer.
2012;59:1180-2.
3. Pomarède R, Finidori J, Czernichow P, Pfister A,
Hirsch JF, Rappaport R. Germinoma in a boy with precocious puberty:
evidence of hCG secretion by the tumoral cells. Childs Brain.
1984;11:298-303.
4. Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock
TI, MacDonald SM. Delayed diagnosis in children with intracranial germ
cell tumors. J Pediatr. 2013;163:1448-53.
5. O’Neil S, Ji L, Buranahirun C, Azoff J, Dhall G,
Khatua S, et al. Neurocognitive outcomes in pediatric and
adolescent patients with central nervous system germinoma treated With a
strategy of chemotherapy followed by reduced-dose and volume
irradiation. Pediatr Blood Cancer. 2011;57:669-73.
6. Khatua S, Dhall G, O’Neil S, Jubran R, Villablanca
JG, Marachelian A, et al. Treatment of primary CNS germinomatous
germ cell tumors with chemotherapy prior to reduced dose whole
ventricular and local boost Irradiation. Pediatr Blood Cancer.
2010;55:42-6.
7. Echevarría ME, Fangusaro J, Goldman S. Pediatric
central nervous system germ cell tumors: A Review. Oncologist.
2008;13:690-9.
8. MacDonald SM, Trofimov A, Safai S, Adams J,
Fullerton B, Ebb D, et al. Proton radiotherapy for pediatric
central nervous system germ cell tumors: early clinical outcomes. Int J
Radiat Oncol Biol Phys. 2011; 79:121-129.
9. Güemes Hidalgo M, Muñoz Calvo MT, Fuente Blanco L,
Villalba Castaño C, Martos Moreno GA, Argente J. Endocrinological
outcome in children and adolescents survivors of central nervous system
tumours after a 5 year follow-up. Ann Pediatr (Barc).
2014;80(6):357-364.
|
|
|
 |
|

