|
|
|
Indian Pediatr 2016;53: 871 -877 |
 |
Maternal Age at Childbirth and Perinatal and
Under-five Mortality in a Prospective Birth Cohort from Delhi
|
|
*# Sikha Sinha,
$Abha Rani
Aggarwal, ‡Clive
Osmond, ‡Caroline
HD Fall, ^Santosh K Bhargava and *Harshpal Singh
Sachdev
From *Sitaram Bhartia Institute of
Science and Research, New Delhi, India; #University
School of Medicine and Para-medical Health Sciences, Guru Gobind Singh
Indraprastha University, Delhi, India; $National
Institute of Medical Statistics, Indian Council of Medical Research, New
Delhi, India; ‡MRC Lifecourse Epidemiology Unit,
University of Southampton, Southampton, UK; and ^Sunder
Lal Jain Hospital, New Delhi, India.
Correspondence to: Prof HPS Sachdev, Senior
Consultant Pediatrics and Clinical Epidemiology, Sitaram Bhartia
Institute of Science and Research, B-16 Qutab Institutional Area, New
Delhi 110 016, India.
Email: [email protected]
Received: April 28, 2016;
Initial review: June 01, 2016;
Accepted: July 07, 2016.
|
Objective: To evaluate the
relationship between maternal age at child birth, and perinatal and
under-five mortality.
Design: Prospective birth cohort.
Setting: Urban community.
Participants: 9169 pregnancies in
the New Delhi Birth Cohort resulted in 8181 live births. These children
were followed for survival status and anthropometric measurements at
birth (+3 days), 3,6,9 and 12 months (7 days), and every 6 months
thereafter until 21 years age. Information on maternal age at child
birth and socio-demographic profile was also obtained.
Outcome measures: Offspring
mortality from 28 weeks gestation till 5 years age.
Results: Offspring mortality
(stillbirths – 5 years; n=328) had a U-shaped association with
maternal age (P<0.001). Compared to the reference group (20-24
years), younger ( £19
years) and older (³
35 years) maternal ages were associated with
a higher risk of offspring mortality (HR: 1.68; 95% CI 1.16, 2.43 and HR
1.48; 95% CI 1.01, 2.16, respectively). In young mothers, the increased
risk persisted after adjustment for socio-economic confounders (maternal
education, household income and wealth; HR 1.51; 95% CI 1.03, 2.20) and
further for additional behavioral (place of delivery) and biological
mediators (gestation and birthweight) (HR 2.14; 95% CI 1.25,3.64).
Similar associations were documented for post-perinatal deaths but for
perinatal mortality the higher risk was not statistically significant (P
>0.05). In older mothers, the increased mortality risk was not
statistically significant (P >0.05) after adjustment for
socio-economic confounders.
Conclusion: Young motherhood is
associated with an increased risk of post-perinatal mortality and
measures to prevent early childbearing should be strengthened.
Key Words: Child mortality, Risk factors,
Teenage pregnancy.
|
|
Reduction of under-five child mortality, the
target of Millennium Development Goal 4 (MDG 4), has shown remarkable
progress globally since 1990, with the highest average annual reduction
rate of 4% during 2005-2013 [1]. Sub-Saharan Africa and South Asia
continue to have the highest under-five mortality burden; India had 49
under-five deaths per 1000 live births in 2013 [2], and is lagging
behind the committed target [3,4]. Perinatal mortality, which includes
stillbirth, has received much less global attention despite being most
common in low- and middle-income countries (LMIC) [5], and has declined
at a slower rate than under-five mortality.
Current interventions for improving child health and
survival focus primarily on medical aspects including immunization, and
improving access to healthcare and illness management, eventhough social
factors are also important. Optimal maternal age at child bearing is one
such undervalued factor [6]. Early marriage and child- bearing are still
quite prevalent in India, especially in rural areas; 18% and 47% are
married before 15 years and 18 years, respectively [7]. If extremes of
maternal age contribute substantially to stillbirths and child
mortality, ensuring an optimal age at childbirth merits greater priority
as an intervention for accelerating progress.
Cross-sectional data suggest that children born to
mothers <20 years of age are at increased risk for perinatal, neonatal
and under-five child mortality [8-12]. However, this existing evidence
has important methodological limitations. There is scant data from
longitudinal cohorts in LMIC [13] exploring the association between
maternal age at childbirth and mortality, particularly in relation to
stillbirths. We, therefore, evaluated the relationship of maternal age
with perinatal and under-five mortality in the New Delhi Birth Cohort
(NDBC), using appropriate statistical techniques and adjustment for
confounders and mediators.
Methods
The NDBC was drawn from a population of 119,799
living in a 12 km 2 area of
south Delhi during 1969-72 [14,15]. 20,755 married women of reproductive
age were recruited and followed regularly every other month to record
menstrual dates. During recruitment, a social worker obtained
information on maternal schooling and age, household structure including
family income, number of family-members, ownership and type of
residence, and sanitation and water supply facilities. Women who became
pregnant were followed every two months initially and on alternate days
from the 37th week of
gestation to determine the pregnancy outcome. There were 9169
pregnancies, resulting in 8181 live births. Survival status and
anthropometric measurements (length and weight) of these babies were
recorded within 72 hrs of birth, at the ages of 3, 6, 9 and 12 months
(±7 days) and every 6 months until 21 years by trained personnel.
Statistical analysis: From the available data,
mortality could be categorized as perinatal (28 weeks gestation to 6
postnatal days), late neonatal (7-28 days), post-neonatal to infant (29
days-1 year), and thereafter at yearly intervals until 5 years. However,
due to small numbers in each of these categories, we used the following
categories in our analysis: (i) all deaths between 28 weeks of
gestation and five years of age (including stillbirths), (ii)
perinatal mortality (28 weeks of gestation-6 days), and (iii)
post-perinatal mortality (7 days- 5 years age).
Data analysis was performed using SPSS version 20.0.
Student’s t-test and Chi square test were used to compare
descriptive statistics between alive and dead cases. Associations of
maternal age at birth with mortality were determined using Cox
Proportional Hazard Model [16]. Maternal age was initially used in a
continuous format and the quadratic term was used to assess the
non-linear associations. Subsequently, it was divided into five groups ( £19,
20-24, 25-29, 30-34 and ³35
years) with 20-24 years (maximum sample size) as the reference category.
The associations between maternal age and offspring
mortality were evaluated in a stepwise manner. Crude analyses adjusted
for the child’s sex, followed by adjustment for confounders, and later
for additional mediators. We included only those potential confounders
and mediators, which were significantly (P<0.05) different
between children who survived and those who died. Confounders included
for adjustment were socio-economic factors (maternal education, per
capita annual household income and household wealth). Household wealth
scores were derived from the 1 st
principal component [17] for the combination of type of housing and
ownership, sanitation, water supply and crowding (number of
people/room); a higher score related to better wealth. The potential
mediators available and considered for additional adjustment were
behavioural (place of delivery and breastfeeding status), and biological
(birth weight and gestation). As breastfeeding status was relevant only
for the post-perinatal deaths, it was not included. The final primary
analyses models were: (i) Model 1- adjusted for sex, (ii)
Model 2- adjusted for sex and socio-economic confounders (maternal
education, household income and household wealth); and (iii)
Model 3- adjusted for sex, socio-economic confounders and mediators
(place of delivery, gestation and birth weight). A sensitivity analysis
was also performed on Model 3 with additional adjustments for
breastfeeding status (only for post-perinatal deaths). Linear and
quadratic associations between maternal age and socio-economic
confounders and mediators were also analyzed.
Results
At the time of recruitment in 1969-1972, 60 percent
of cohort families had an income above 50 rupees per month (national
average, 28) and only 15 percent of parents were illiterate (national
average, 66). Nevertheless, 43% of families lived in one room. Hindus
were the majority religious group (84%) [15]. Information on maternal
age at child birth was available for 5886 subjects (mean (SD) age 25.9
(5.3) years). All of them were married and 67% of them living in masonry
buildings with good water supply and sanitation facilities. Only 31.5%
of the mothers had received 10 or more years of education.
There were 328 deaths reported up to 5 years of age
including stillbirths, with no significant sex differences (Table
I). Most deaths (84%) had occurred by 1 year of age, with neonatal
to infant (41.1%), perinatal (29.0%) and late neonatal (13.7%) deaths
being the major contributors. Demographic and birth characteristics
among those censored (alive) and those who died are compared in
Web Table I. Considering all deaths, children who had died were
born smaller and at an earlier gestation than survivors. Their mothers
had less education and poorer housing, water supply and sanitation
facilities, and lower per capita annual household income and household
wealth scores. However, there were no differences in mean maternal age
at childbirth and birth order. An analysis restricted to post-perinatal
and perinatal deaths, yielded similar findings. Predominant
breastfeeding was nearly universal (98.9% at birth and 91.5% at 3
months) but practised more often in survivors. However, for perinatal
deaths, the place of delivery and most of the socio-economic variables
were not significantly different, except for household income and
house-ownership.
TABLE I Sex-wise Mortality Distribution
|
Mortality period |
Male |
Female |
Total |
|
Perinatal (28 wk gestation-6 d) |
52 (33.3) |
43 (25.0) |
95 (29.0) |
|
Late neonatal (7 d-28 d) |
21 (13.5) |
24 (13.9) |
45 (13.7) |
|
Post-neonatal infant (29 d-1 y) |
64 (41.0) |
71 (41.3) |
135 (41.1) |
|
1-2 y |
10 (6.4) |
23 (13.4) |
33 (10.0) |
|
2-3 y |
5 (3.2) |
5 (2.9) |
10 (3.1) |
|
3-5 y |
4 (2.6) |
6 (3.5) |
10 (3.1) |
|
Total |
156 |
172 |
328 |
|
All values in No.(%). No statistically significant sex
differences. |
All the socio-economic confounders (maternal
education, household income and household wealth), and mediators (place
of delivery, gestation and birthweight) had inverted U-shaped
relationship with maternal age (P £0.001
for quadratic term) (Web Table II). Both younger and older
age of mothers was associated with lower education, household income,
wealth, birthweight and gestation, and less likely to deliver in the
healthcare services. Maternal age was unrelated to breastfeeding status.
Offspring mortality (stillbirths – 5 years) had a
significant U-shaped relationship with maternal age (P<0.001),
which persisted after adjustment for socio-economic status confounders (P=0.003)
and mediators (P=0.018) (Web Table III). There were
similar associations, of borderline significance in the
mediator-adjusted model (P=0.07), for post-perinatal deaths.
However, for perinatal deaths there was no evidence of a significant (P>0.05)
quadratic association.
TABLE II Association Between Different Maternal Age-groups and Offspring Mortality
|
Variables |
Model 1 Hazard ratio |
Model 2 Hazard ratio |
Model 3 Hazard ratio |
|
(95% CI) (P value) |
(95% CI) (P value) |
(95% CI) (P value) |
|
All deaths |
|
Number of deaths/total sample (deaths + censored) Maternal age
(years) |
328/5886 |
316/5478 |
156/4154 |
|
-19 |
1.68 (1.16; 2.43) (0.006) |
1.51 (1.03; 2.20) (0.033) |
2.14 (1.25; 3.64) (0.005) |
|
20-24 |
Reference |
Reference |
Reference |
|
25-29 |
1.00 (0.76; 1.31) (0.982) |
0.94 (0.71; 1.24) (0.655) |
1.34 (0.88; 2.05) (0.178) |
|
30-34 |
1.00 (0.71; 1.40) (0.990) |
0.77 (0.54; 1.09) (0.140) |
1.02 (0.59; 1.74) (0.956) |
|
35+ |
1.48 (1.01; 2.16) (0.043) |
0.99 (0.66; 1.48) (0.968) |
1.74 (1.02; 2.97) (0.042) |
|
Perinatal deaths |
|
Number of deaths/total sample (deaths + censored) Maternal age
(years) |
95/5886 |
91/5478 |
29/4154 |
|
-19 |
1.51 (0.78; 2.92) (0.219) |
1.42 (0.72; 2.83) (0.312) |
1.22 (0.32; 4.63) (0.775) |
|
20-24 |
Reference |
Reference |
Reference |
|
25-29 |
0.90 (0.54; 1.48) (0.667) |
0.84 (0.50; 1.40) (0.498) |
1.07 (0.40; 2.83) (0.891) |
|
30-34 |
0.91 (0.49; 1.69) (0.759) |
0.77 (0.40; 1.45) (0.410) |
0.85 (0.22; 3.31) (0.817) |
|
35+ |
0.93 (0.41; 2.09) (0.852) |
0.61 (0.25; 1.49) (0.280) |
1.73 (0.51; 5.90) (0.380) |
|
Post-perinatal deaths |
|
Number of deaths/total sample (deaths + censored) Maternal age
(years) |
233/5483 |
225/5080 |
127/3894 |
|
-19 |
1.77 (1.13; 2.75) (0.012) |
1.57 (1.00; 2.46) (0.052) |
2.39 (1.33; 4.28) (0.003) |
|
20-24 |
Reference |
Reference |
Reference |
|
25-29 |
1.05 (0.75; 1.46) (0.790) |
0.98 (0.70; 1.38) (0.911) |
1.38 (0.86; 2.22) (0.180) |
|
30-34 |
1.04 (0.70; 1.56) (0.845) |
0.76 (0.50; 1.16) (0.209) |
1.05 (0.59; 1.89) (0.862) |
|
35+ |
1.73 (1.13; 2.67) (0.013) |
1.14 (0.72; 1.79) (0.580) |
1.69 (0.93; 3.08) (0.087) |
|
Model 1: adjusted for sex; Model 2: adjusted for sex,
socio-economic confounders (maternal education, household income
and wealth); and Model 3: adjusted for sex, socio-economic
confounders and biological mediators (place of delivery,
gestation and birth weight). |
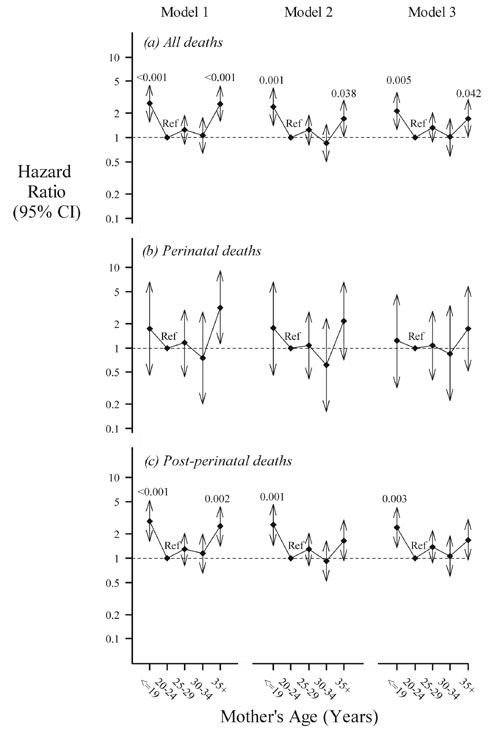
All deaths (stillbirths and mortality till five years
of age): Table II depicts the risk of offspring
mortality across the five maternal age groups. In comparison to mothers
aged 20-24 years, younger ( £19
years) and older (³35
years) maternal ages were associated with higher offspring mortality
(stillbirth – 5 years) (HR:1.68; 95% CI 1.16, 2.43 and HR 1.48; 95% CI
1.01, 2.16, respectively). After adjustment for socio-economic
confounders, this higher risk persisted for younger mothers (HR 1.51;
95% CI 1.03, 2.20) but not for older mothers (HR 0.99; 95% CI 0.66,
1.48). On further adjustment for mediators, offspring of both younger
and older mothers had a higher risk of mortality (HR 2.14; 95% CI 1.25,
3.64 and HR 1.74; 95% CI 1.02, 2.97, respectively). In order to estimate
the change in effect size of the association with additional confounder
and mediator adjustments (which led to reductions in sample size),
models 1 and 2 were run on the available sample for the fully adjusted
model 3 (Fig. 1). The hazard ratios for both younger and
older mothers were sequentially attenuated from the crude to the fully
adjusted models. The mothers available for fully adjusted model 3 (after
reduction in sample size) were comparatively educated and had marginally
higher household income and wealth score.
 |
|
Fig. 1 Hazard ratio for mortality
across different age groups of maternal age at childbirth (a)
all deaths till five years including stillbirths (Number of
deaths/total sample: 156/4154); (b) Perinatal deaths (Number of
deaths/total sample: 29/4154); (c) Post-perinatal deaths (Number
of deaths/total sample: 127/3894). (Model 1: adjusted for
sex; Model 2: further adjusted for socio-economic confounders
and Model 3: further adjusted for mediators (type of delivery,
gestation and birth weight). The bars represent 95% confidence
interval for the hazard ratio and figures at the top of the bars
are P value for significant age groups. Ref: Reference age
group.
|
Post-perinatal or perinatal deaths: A similar
pattern was found for post-perinatal mortality; the increased risk being
statistically significant (P<0.05) for all three models in
younger but not older mothers. The attenuation pattern was similar for
perinatal deaths but the increased risk was not statistically
significant. There were no instances for which the point estimate in one
time interval was outside the 95% confidence for the other time
interval, thereby suggesting that the effect sizes were similar or
hazard was proportional in both perinatal and post-perinatal categories.
On sensitivity analyses (data not presented), the
mortality risk for younger and older mothers remained similar after
additional adjustments for birth-order (all three death categories) and
breastfeeding (post-perinatal deaths).
Discussion
In this prospective cohort study, offspring of young
(<20 years) mothers had an increased risk of mortality from the
perinatal period up to age five years, primarily after the early
neonatal period. An apparently similar dis-advantage in older (>35
years) mothers was principally a reflection of their adverse
socio-economic profile.
Persistence of a higher overall mortality risk in
children of young mothers, despite adjustments for confounders and
mediators, suggests a causal relationship. Similar effects were evident
for post-perinatal deaths but not for perinatal mortality. This could
either reflect a true biological difference or insufficient statistical
power for the perinatal mortality component, which showed broadly
similar associations (29-95 deaths in various models). The
confounder-adjusted association for post-perinatal mortality was further
attenuated after the introduction of mediators and, except
breastfeeding, the other three biological and behavioural factors (place
of delivery, gestation and birth weight) were significantly related to
young maternal age. The increased risk appears to be partly operating
through lower birth weight and gestation [6], and less utilization of
health care services (home delivery). These factors; however, are of
limited relevance for the stillbirth component of perinatal mortality as
the event is likely to determine the birth weight, gestation and access
to health care rather than the converse. In contrast, the increased
overall mortality risk in older mothers was not evident after
socio-economic adjustments. Older maternal age may thus not biologically
predispose the offspring to higher mortality, and older mothers are also
likely to be more experienced in child care practices. In a recent
meta-analysis of five birth cohorts from LMIC (of which NDBC was one)
children of older mothers had a higher risk of preterm birth, but had
better nutritional status and schooling after similar confounder
adjustment [6]. Older mothers available for the fully adjusted model 3
had higher education and wealth score, which along with a lower sample
size could explain the observed statistically significant associations.
Earlier cross-sectional data, including pooled
analyses from 118 demographic and health surveys conducted between 1990
and 2008 in 55 low and middle income countries (LMIC), also documented a
higher risk of perinatal, neonatal, infant and under-five mortality in
young mothers [8-12,18-23]. It is suggested that this risk may operate
through both biological and social mechanisms. Some studies also
documented a higher risk in older mothers or J or U shaped association,
particularly for unadjusted models [18,24]. However, this evidence has
important limitations: (a) Cross-sectional design and variation
in context and time period; (b) Sub-optimal confounder
adjustments; (c) Non-linear relationships have been rarely
explored; and (d) Prospective data collection, to minimize bias,
is mostly restricted to developed countries. Three population-based
cohorts in Brazil (1982, 1993 and 2004) observed an increased risk of
post-neonatal infant mortality (confounder adjusted OR 1.6; 95% CI 1.2,
2.1) in children of young (<20 years) mothers but not for stillbirths,
perinatal deaths or neonatal mortality [13]. Further adjustment for
mediating variables (place of delivery, gestation and birth weight) led
to the disappearance of the excess of post-neonatal mortality. It was
concluded that social and environmental factors may be more important
than biological immaturity for this increased mortality. However, in our
data, the increased risk for post-perinatal deaths persisted even after
confounder and mediator adjustment, suggesting a causal relationship.
These observed differences, among other factors, could relate to
contextual variability, baseline mortality risk, social characteristics
of young mothers, social and health care support systems and
methodological differences (surveillance versus prospective
cohort follow up, including or excluding mothers
³30 years and
restricting outcomes to infant or under-five mortality). We thus
hypothesize that young maternal age predisposes the offspring to higher
post-perinatal mortality, which only partly operates through
socio-economic deprivation and biological-behavioural mediators (lower
birthweight and gestation, and poorer access to healthcare); the
additional precise biological mechanisms need further exploration.
Strengths of our study are a large sample size,
prospective community-based recording of confounders, mediators and
outcomes until five years age from a South Asian setting, and
appropriate analyses. The following limitations also merit
consideration: (i) the relevance of four decades old data for
contemporary programmes could be questioned. However, the findings have
important programmatic implications for several regions in the country
that even now have similar fertility, mortality, poor socio-economic,
water supply and sanitation and health access indicators. Further, there
was no evidence of secular changes in associations in data spread over
2-3 decades. [13,18]; (ii) data are missing for some variables;
however, most of this pertains to mediators rather than confounders and
this is a familiar scenario in large prospective cohort studies from
LMIC; (iii) there may be some residual unadjusted confounding; (iv)
a separate category of early neonatal deaths was not available for
analysis. In community settings in India, it is challenging to discern a
live newborn from a stillbirth within the first day of delivery.
Offspring of teenage mothers in LMIC not only have
poorer child survival, but are also disadvantaged at birth and during
childhood, and have reduced human capital [6]. Measures to prevent young
motherhood are currently underrated as public health interventions;
these should receive greater prominence and investments in the proposed
child health and survival agenda [25]. Teenage marriages and pregnancies
are declining in India [26,27]. However, as per latest national
estimates, 32% of all women and 40% of those illiterate are married
before 18 years [26]; the intervention thus still retains importance,
particularly in rural and tribal regions. Further, greater care and
support is necessitated for their vulnerable children in public health
programs. It would be unethical to conduct randomized controlled trials
on this subject. However, operational and behavioural research to
prevent young motherhood in different contexts is desirable. Pooled
analyses from recent similar cohorts in LMIC could confirm the utility
of this intervention with improvements in access to health care.
In conclusion, children of teenage mothers are at an
increased risk of post-perinatal mortality and measures to prevent young
motherhood should be strengthened.
Contributors: SS, ARA, HPS, CHDF, SKB:
conceptualised the study. SS, ARA, CO, HPS: analyzed the data. SS:
drafted the initial manuscript. All authors contributed to the critical
revision of the article.
Funding: Indian Council of Medical Research for
supporting Ms. Sikha Sinha through the Senior Research Fellowship
Scheme. The original cohort studies were supported by the National
Center for Health Statistics, USA and the Indian Council of Medical
Research.
Competing interest: None stated.
|
What is Already Known?
• Cross-sectional analyses, often with
inadequate confounder adjustments, suggest that young motherhood
is associated with perinatal, neonatal and under-five mortality
What This Study Adds?
• This prospective birth cohort data with
confounder and mediator adjustments indicate that children of
teenage mothers are at an increased risk of post-perinatal
mortality, and measures to prevent young motherhood should be
strengthened. An apparently similar disadvantage in older (>35
years) mothers is principally a reflection of their adverse
socio-economic profile.
|
References
1. You D, Hug L, Ejdemyr S, Idele P, Hogan D, Mathers
C, et al. Global, regional, and national levels and trends in
under-5 mortality between 1990 and 2015,with scenario-based projections
to 2030: A systematic analysis by the UN Inter-agency Group for Child
Mortality Estimation. Lancet. 2015;386: 2275-86.
2. SRS Statistical Report, September 2014, Volume 49
No. 1. Available from: http://censusindia.gov.in/vital_statistics/SRS_Bulletins/SRS%20Bulletin%20-Sepetember%
202014.pdf. Accessed March 30, 2016.
3. Paul VK, Sachdev HS, Mavalankar D, Ramachandran
P, Sankar MJ, Bhandari N, et al. Reproductive health, and child
health and nutrition in India: meeting the challenge.
Lancet. 2011;377:332-49.
4. IGME Report 2015 Child Mortality Final Unicef.
Available from:
http://www.childmortality.org/files_v20/download/IGME%20report%202015%20child%
20mortality%20final.pdf. Accessed March 30, 2016.
5. Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers
C, Hogan D, et al. ; Lancet Ending Preventable Stillbirths Series
study group; Lancet Stillbirth Epidemiology investigator group.
Stillbirths: rates, risk factors, and acceleration towards 2030.
Lancet. 2016;387:587-603.
6. Fall CH, Sachdev HS, Osmond C, Restrepo-Mendez
MC, Victora C, Martorell R, et al.; COHORTS investi-gators.
Association between maternal age at childbirth and child and adult
outcomes in the offspring: a prospective study in five low-income and
middle-income countries (COHORTS collaboration). Lancet Glob Health.
2015; 3:e366-77.
7. The State of the World’s Children 2015: Executive
Summary. http://www.unicef.org/publications/files/SOWC _
2015_Summary_and_Tables.pdf. Accessed June 6, 2016.
8. Markovitz BP, Cook R, Flick LH, Leet TL.
Socio-economic factors and adolescent pregnancy outcomes: Distinctions
between neonatal and post-neonatal deaths? BMC Public Health. 2005;5:79.
9. Chen XK, Wen SW, Fleming N, Yang Q, Walker MC.
Increased risks of neonatal and postneonatal mortality associated with
teenage pregnancy had different explanations. J Clin Epidemiol. 2008;61:688-94.
10. Kapoor RK, Srivastava AK, Misra PK, Sharma B, Thakur
S, Srivastava KI, et al. Perinatal mortality in urban slums in
Lucknow. Indian Pediatr. 1996;33:19-23.
11. Raj A, Saggurti N, Winter M, Labonte A, Decker
MR, Balaiah D, et al. The effect of maternal child marriage on
morbidity and mortality of children under 5 in India: cross sectional
study of a nationally representative sample. Br Med J. 2010;340:b4258.
12. Singh R, Tripathi V. Maternal factors
contributing to under-five mortality at birth order 1 to 5 in India: a
comprehensive multivariate study. Springerplus. 2013; 2:284.
13. Restrepo-Méndez MC, Barros AJ, Santos IS, Menezes
AM, Matijasevich A, Barros FC, et al. Childbearing during
adolescence and offspring mortality: findings from three
population-based cohorts in southern Brazil. BMC Public
Health. 2011;11:781.
14. Richter LM, Victora CG, Hallal PC, Adair
LS, Bhargava SK, Fall CH, et al; COHORTS Group. Cohort profile:
the Consortium of Health-Orientated Research in Transition-ing
Societies. Int J Epidemiol. 2012;41:621-6.
15. Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy
R, Barker DJ, et al. Relation of serial changes in childhood
body-mass index to impaired glucose tolerance in young adulthood. N Engl
J Med. 2004;350:865-75.
16. Bradburn MJ, Clark TG, Love SB, Altman DG.
Survival analysis part II: multivariate data analysis — an introduction
to concepts and methods. Br J Cancer. 2003;89:431-6.
17. Vyas S, Kumaranayake L. Constructing
socio-economic status indices: how to use principal components analysis.
Health Policy Plan. 2006;21:459-68.
18. Finlay JE, Ozaltin E, Canning D. The association
of maternal age with infant mortality, child anthropometric failure,
diarrhoea and anemia for first births: evidence from 55 low-and
middle-income countries. BMJ Open. 2011;1:e000226.
19. Haldre K, Rahu K, Karro H, Rahu M. Is a poor
pregnancy outcome related to young maternal age? A study of teenagers in
stonia during the period of major socio-economic changes (from 1992 to
2002). Eur J Obstet Gynecol Reprod Biol. 2007;131:45-51.
20. Chen XK, Wen SW, Fleming N, Demissie K, Rhoads
GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large
population based retrospective cohort study. Int J Epidemiol.
2007;36:368-73.
21. Gilbert W, Jandial D, Field N, Bigelow P,
Danielsen B. Birth outcomes in teenage pregnancies. J Matern Fetal
Neonatal Med. 2004;16:265-70.
22. Olausson PO, Cnattingius S, Haglund B. Teenage
pregnancies and risk of late fetal death and infant mortality. Br J
Obstet Gynaecol. 1999;106:116-21.
23. Cowden AJ, Funkhouser E. Adolescent pregnancy,
infant mortality, and source of payment for birth: Alabama residential
live births, 1991-1994. J Adolesc Health. 2001;29:37-45.
24. Golding J, Greenwood R, McCaw-Binns A, Thomas P.
Associations between social and environmental factors and perinatal
mortality in Jamaica. Paediatr Perinat Epidemiol. 1994;8:17-39.
25. Were WM, Daelmans B, Bhutta Z, Duke T, Bahl R, Boschi-Pinto
C, et al. Children’s health priorities and interventions. Br Med
J. 2015;351:h4300.
26. C-6. Ever Married and Currently Married
Population by Age at Marriage, Duration of Marriage and Educational
Level - 2011 (India and States/UTs/District level). Available from:
http://www.censusindia.gov.in/2011 census/C-series/C-6.html(DDW-0000C-06.XLSX).
Accessed June 6, 2016.
27. NFHS 4 Factsheet. Available from:
http://rchiips.org/NFHS/factsheet_NFHS-4.shtm. Accessed March 30,
2016.
|
|
|
 |
|

