|
|
|
Indian Pediatr 2015;52: 847 -851 |
 |
Efficacy and Safety of Drotaverine
Hydrochloride in Children with Recurrent Abdominal Pain: A
Randomized Placebo Controlled Trial
|
|
Manish Narang, Dheeraj Shah and Hina Akhtar
From the Division of Pediatric Gastroenterology,
Hepatology and Nutrition, Department of Pediatrics, University College
of Medical Sciences (University of Delhi) and Guru Teg Bahadur Hospital,
Delhi, India.
Correspondence to: Dr Dheeraj Shah, Associate
Professor, Department of Pediatrics, UCMS and GTB Hospital, Dilshad
Garden, Delhi 110 095, India.
Email: [email protected]
Received: January 02, 2015;
Initial review: March 13, 2015;
Accepted: July 15, 2015.
CTRI/2012/07/002765
|
Objectives: To evaluate the efficacy and
safety of Drotaverine hydrochroride in children with recurrent abdominal
pain.
Design: Double blind, randomized
placebo-controlled trial.
Setting: Pediatric Gastroenterology clinic of a
teaching hospital.
Participants: 132 children (age 4-12 y) with
recurrent abdominal pain (Apley Criteria) randomized to
receivedrotaverine (n=66) or placebo (n=66) orally.
Intervention: Children between 4-6 years of age
received 10 mL syrup orally (20 mg drotaverine hydrochloride or placebo)
thrice daily for 4 weeks while children >6 years of age received one
tablet orally (40 mg drotaverine hydrochloride or placebo) thrice daily
for 4 weeks.
Outcome Measures: Primary: Number of
episodes of pain during 4 weeks of use of drug/placebo and number of
pain-free days. Secondary: Number of school days missed during
the study period, parental satisfaction (on a Likert scale), and
occurrence of solicited adverse effects.
Results: Reduction in number of episodes of
abdominal pain [mean (SD) number of episodes 10.3 (14) vs 21.6
(32.4); P=0.01] and lesser school absence [mean (SD) number of
school days missed 0.25 (0.85) vs 0.71 (1.59); P=0.05] was
noticed in children receiving drotaverine in comparison to those who
received placebo. The number of pain-free days, were comparable in two
groups [17.4 (8.2) vs 15.6 (8.7); P=0.23]. Significant
improvement in parental satisfaction score was noticed on Likert scale
by estimation of mood, activity, alertness, comfort and fluid intake.
Frequency of adverse events during follow-up period was comparable
between children receiving drotaverine or placebo (46.9% vs
46.7%; P=0.98),
Conclusion: Drotaverine hydrochloride is an
effective and safe pharmaceutical agent in the management of recurrent
abdominal pain in children.
Keywords: Abdominal pain, Parental satisfaction, Treatment.
|
|
Recurrent abdominal pain (RAP) is one of the most
common chronic pain conditions of
childhood. Between 4% to 25% of school-age
children complain of RAP of sufficient severity to interfere with daily
activities [1-4]. Most common cause of recurrent abdominal pain in
children is functional abdominal pain (FAP) which may be caused by
alterations of homeostatic reflexes in gut-brain axis that is involved
in control of gastrointestinal functions. This can be associated with
dysregulations in intestinal secretions, motility, blood flow and
afferent sensitivity [5]. This may respond to cognitive behavioral
therapy, but medications are frequently prescribed for relief of pain
[6].
Drotaverine, a selective inhibitor of
phospho-diesterase (PDE) isoenzyme IV, has been found to be useful in
spastic and motility disorders of the smooth muscle in adults [7-9].
However, good quality data about its efficacy in children are lacking.
Drotaverine is the most commonly used off-label medication in Europe for
alimentary tract problems in preschool and school children [10].
Although drotaverine is frequently used as spasmolytic in children, its
efficacy in control of functional abdominal pain – the most common
chronic pain condition – has not been evaluated in children. As RAP is a
chronic condition requiring frequent doses of the drug, the safety over
prolonged/repeated use also needs to be documented. The present
randomized placebo-controlled trial was conducted to assess the efficacy
and safety of drotaverine in children with recurrent abdominal pain.
Methods
This double-blind, randomized placebo-controlled
trial was conducted at Pediatric Gastroenterology and Hepatology Clinic
of a tertiary care hospital in Northern India catering mainly to urban
poor population. The study was conducted over 12 months period ending
September 2013. The study protocol was approved by Institutional Ethics
Committee (Human Research) of GTB Hospital, Delhi. Written informed
consent was obtained from parents, and assent was taken from children
aged ł7 years.
Children aged between 4 to 12 years with recurrent
abdominal pain, defined as at least three episodes of pain interfering
with normal activities within a three month period [1], were screened
for potential inclusion into the study. Patients were excluded from the
study if they had organic etiology (e.g., cholelithiasis, nephro/urolithiasis,
acute pancreatitis, viral hepatits, previous abdominal surgery) of
abdominal pain (as apparent from history, clinical examination or
investigations), cognitive-developmental delay, cerebral palsy, previous
abdominal surgery, acute illness (fever, diarrhea or respiratory tract
infection in last 3 days), known immunodeficiency, or chronic cardiac,
hepatic or renal disease.
Initial evaluation included a detailed medical
history and complete physical and systemic examination. Blood
investigations in all patients included hemoglobin, total and
differential counts, erythrocyte sedimentation rate, serum bilirubin,
alanine aminotransferase, serum albumin, urea, sodium and potassium.
Microscopy and culture of urine, stool examination, plain abdominal
radiograph, and ultrasonography of abdomen were also performed in all
eligible children.
Enrolled children were randomly assigned to either
receive the drug or the placebo with the use of a randomization list
using computer-generated block randomization with variable block size.
Stratification was done equally for ages 4-6 years and for >6 years.
Allocation concealment was done in sealed opaque envelopes using six
codes to avoid guessing of code; bottles were labelled with one of these
codes. Participants, their parents, investigators and outcome assessors
were blind to the treatment assigned. The drugs and placebo were
packaged identically, and were similar in appearance, taste and smell.
The placebo contained identical components to those in the active
treatment group, with the exception of drotaverine hydrochloride.
Randomization was done by a person not directly involved in the study.
The code was kept in a sealed envelope in a locked cupboard. This code
was broken only after complete data entry and cleaning.
For children aged between 4 to 6 years, 10 mL of the
drug suspension or placebo (providing 20 mg of drotaverine hydrochloride
in those receiving drug) was administered orally thrice a day for
duration of four weeks. For children aged more than six years, one
tablet containing 40 mg drotaverine hydrochloride or placebo was given
orally thrice a day for a period of four weeks. If the child encountered
an episode of pain, the next dose was preponed if it was due in next two
hours. One additional dose was given if he/she had not received the
maintenance dose in last one hour or if next dose was not due in next
two hours.
On a daily basis from week 1 to week 4, patients
recorded the frequency/severity of pain and school absence in a
structured diary provided by investigators. To assess the severity of
pain, a combination of the self-reported visual analog scale (VAS) [11]
and the Faces Pain Scale (FPS) [12] were used. These scales were printed
in the patient diary for assessment by parents during the episode of
pain. The caregiver satisfaction was assessed on a Likert scale based on
their perception of child’s mood, activity, alertness, oral intake and
comfort. The parent’s response was rated on a 5-point scale ranging from
bad (1) to completely normal (5); subscale scores were computed by
calculating the mean rating for each response. Higher scores indicated
higher level of parental satisfaction. Number of school days missed
during treatment due to pain was obtained by parent report. The question
asked was ‘Has the child missed school due to abdominal pain during last
week’?
Enrolled children were called weekly in the clinic to
examine their symptom diary. Entries were copied from the patient diary
to the case record form. Any missing entry into the diary was clarified
during each visit. The drug/placebo bottles (containing tablets or
syrup) were dispensed on a weekly basis, the supply being sufficient to
last for 10 days to take care of any additional doses required.
Compliance to treatment was assessed by measuring/counting the remaining
drug. All empty containers were preserved till the end of the study.
Children missing more than 20% of the medication were considered
non-compliant. Adverse events (both solicited and unsolicited) were
monitored throughout the study in a symptom diary.
Primary outcome measures included number of episodes
of pain during 4 weeks of use of drug/placebo and number of pain-free
days. Secondary outcome measures included number of school days
missed during the study period, parental satisfaction (on a Likert
scale) and occurrence of solicited adverse effects (vertigo, headache,
nausea or vomiting).
A sample size of 110 (55 in each group) was
calculated to be sufficient to detect 15% difference in number of pain
episodes during the 4 week observation period in two groups assuming a
coefficient of variation of 30%, with power of 80% and alpha of 0.05.
Accounting for 15% attrition, we planned to enroll 132 (66 in each
group) children.
Statistical analysis: Analysis was
performed as per protocol analysis. Details of patients who were
lost to follow-up were compared in the two groups. The mean number of
episodes of pain and number of pain-free days were compared in two
groups by Student-t test. Frequencies were compared using
Chi-square test or Fischer Exact test, as applicable. P value
<0.05 was considered as significant. Data were entered into Microsoft
Excel spreadsheet and analyzed by SPSS Version 17.0 statistical
software.
Results
Two-hundred-four participants with recurrent
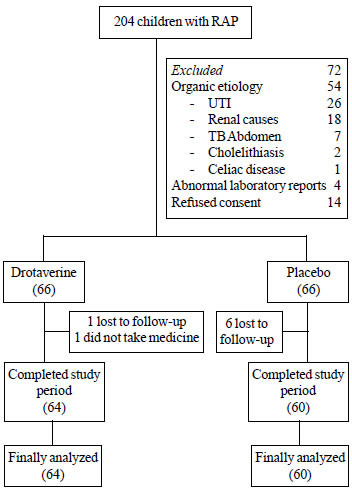
abdominal pain were screened for inclusion in the study. Fig.1
shows the flow of participants through the study. There were no
significant differences between the groups in baseline characteristics (Table
I).
 |
|
Fig. 1 Study flow chart.
|
TABLE I Baseline Demographic Characteristics of Children Receiving Drotaverine or Placebo
|
Drotaverine group |
Placebo group |
|
(n=66) |
(n=66) |
|
Age, y |
7.1 (2.1) |
7.4 (2.6) |
|
Boys; No. (%) |
33 (50) |
39 (59) |
|
Weight, kg |
20.4 (6.2) |
21.1 (6.1) |
|
Height, cm |
115.9 (15.0) |
115.9 (15.7) |
|
Duration of pain, mo |
10 (10.0) |
9.6 (10.2) |
|
Severity (VAS) of a typical episode, score |
5.5 (1.5) |
5.9 (1.9) |
|
*Site of Pain; No (%) |
|
Umbilical |
53 (80.3) |
55 (83.3) |
|
Epigastric |
10 (15.2) |
10 (15.2) |
|
Others |
8 (12.1) |
(6.1)4 |
|
*Some children had pain at more than one site; All values
are in Mean (SD) unless specified. |
There was a significant reduction in episodes of
abdominal pain in children receiving drotaverine in comparison to those
receiving placebo (Table II). Frequency of children
missing school days were significantly lesser in drotaverine group as
compared to placebo group. A total of 8 additional doses (in 4 children)
were consumed by children receiving drotaverine as against 21 doses (in
10 children) in placebo group. The number of patients requiring
additional drug doses, number of additional doses and mean number of
additional drug doses in drotaverine group and placebo group were not
significantly different. Frequency of adverse events was comparable
between two groups. Most of the local and general adverse events were
intercurrent illnesses such as upper respiratory infection or fever, not
causally related to the study drug. All the adverse events resolved
before completion of the study without sequela. There were no deaths or
any serious adverse events. One patient in drotaverine group developed
urticaria which required discontinuation of the drug.
TABLE II Outcome Variables in Each Treatment Group
|
Outcome |
Drotaverine |
Placebo |
P value |
|
(n= 64) |
(n=60) |
|
|
Pain episodes |
10.3 (14) |
21.6 (32.4) |
0.015 |
|
Pain-free days |
17.4 (8.2) |
15.6 (8.7) |
0.234 |
|
School days missed |
0.25 (0.85) |
0.71 (1.59) |
0.054 |
|
Any school absence; No. (%) |
6 (9.4) |
14 (23.3) |
0.034 |
|
Patients with additional |
4 (6.2) |
10 (16.7) |
0.090 |
|
dose requirement; No. (%) |
|
|
|
|
Episode of pain during |
7 (10.9) |
3 (5.0) |
0.326 |
|
follow-up period; No. (%) |
|
|
|
|
Adverse events; No. (%) |
30 (46.9) |
28 (46.7) |
0.981 |
|
*Adverse Events; No. (%) |
53 |
43 |
0.138 |
|
Fever |
10 |
6 |
|
|
Cough |
8 |
7 |
|
|
Cold |
5 |
4 |
|
|
Vomiting |
7 |
8 |
|
|
Nausea |
6 |
2 |
|
|
Giddiness |
4 |
2 |
|
|
Diarrhea |
4 |
3 |
|
|
Macular rash |
4 |
1 |
|
|
Headache |
3 |
5 |
|
|
Uricaria |
1 |
0 |
|
|
Eating poorly than usual |
1 |
3 |
|
|
Epistaxis |
0 |
1 |
|
|
Black Stools |
0 |
1 |
|
|
*Some children had more than one adverse event; All values
are in Mean (SD) unless specified. |
The parental satisfaction scores are compared
Table III. The overall mean scores for mood, activity,
alertness, comfort and fluid intake were higher in the drotaverine than
the control group during the 4 weeks of treatment.
TABLE III Parental Satisfaction Score (After 4 Weeks of Treatment) in Each Treatment Group
|
Outcome |
Drotaverine |
Placebo |
|
Mean (SD) |
Mean (SD) |
|
Mood |
3.7 (0.7) |
3.3 (0.9) |
|
Activity |
3.7 (0.7) |
3.4 (0.9) |
|
Alertness |
3.8 (0.8) |
3.5 (0.9) |
|
Comfort |
3.7 (0.8) |
3.4 (0.9) |
|
Fluid Intake |
4.0 (0.7) |
3.7 (0.8) |
|
P<0.05 for all comparisons |
Discussion
In this randomized controlled trial on children with
non-organic recurrent abdominal pain, we documented that drotaverine
given orally for four weeks results in fewer episodes of abdominal pain
and school absence, and improves parental satisfaction as compared to
placebo group. No significant drug-related adverse effects were
observed.
There were several limitations to this study. First,
our definition of recurrent abdominal pain was based on Apley’s criteria
[1], which considers recurrent abdominal pain as a single entity, and
not as per the new Rome III criteria which considers this too wide for
useful application and sub-classifies functional abdominal pain by
symptomatology and cause [13]. However, a Cochrane review concluded that
it remains unclear the extent to which separating children into
sub-groups (as per Pediatric Rome Criteria II of 1999) [14] defines
groups who have different psychological or pathophysiological mechanisms
underlying their symptoms or whether they are likely to respond
differently to interventions [6]. Second, the drug was administered by
parents thrice daily while children were examined once weekly by
clinicians in the study clinic. However, we ensured compliance by
counting the tablets or measuring the volume of remaining drug at every
visit. Evaluation of pain was done by parents who are likely to vary in
the way they engage in certain type of responses. No biochemical
monitoring of adverse events was done. The drug was given on regular
basis rather than as-and-when required basis to assess safety and
acceptability of repeated doses, which may not be always required in a
clinical setting. The study was carried out in recurrent functional
abdominal pain with other causes of abdominal pain not being addressed.
Single-center trial and short follow-up period are the other limitations
of the study. Strengths of our study were: randomized placebo-controlled
trial design, detailed work-up to exclude other causes of abdominal
pain, and evaluation of functional outcomes such as episodes of
abdominal pain, school absenteeism and parental satisfaction.
A Cochrane review assessing effectiveness of
medication in 5-18 years old school age children with RAP concluded that
there is paucity of placebo-controlled trials for all of the drugs
recommended for use in children with RAP [6]. However, individual
studies have documented efficacy of other treatments in children with
functional abdominal pain [15-18]. Evidence is inconclusive for some
other treatment modalities such as H 2-receptor
antagonists [19], fiber supplement intake or lactose free diet in
children with RAP [20,21]. There is a paucity of comparative efficacy
data for drotaverine in children. However, in adults, drotaverine has
proven to be effective antispasmodic in renal colic [22,23] and
irritable bowel syndrome [24], with no serious side effects. In the
current study, drotaverine was associated with fewer episodes of
abdominal pain during its regular use. The precise mechanism by which
drotaverine can relieve abdominal pain is due to its antispasmodic
properties, which is devoid of anticholinergic activity. It acts mainly
by inhibiting type IV PDE, leading to an increase in intracellular
cyclic AMP and cyclic GMP leading to smooth muscle relaxation.
We conclude that drotaverine hydrochloride is an
effective and safe pharmaceutical agent in the management of recurrent
abdominal pain of childhood. Further studies of its efficacy in organic
abdominal pain conditions of childhood are desirable. Future studies
should address the issue of its efficacy when given on as-and-when
required basis, along with biochemical monitoring of any adverse
effects.
Contributors: MN: Data collection and manuscript
writing; HA: Data collection and manuscript revision; DS: Study
conception, data collection and analysis, and critical review of
manuscript for intellectual content. All authors approved the final
version of the paper.
Funding: Walter Bushnell Pvt. Ltd. The grant and
medications/placebo for the conduct of submitted work was provided by
the company. The company had no role in research design, data
collection, data analysis or manuscript preparation.
Competing interest: No financial relationship of
authors with any organization that might have interest in the submitted
work.
|
What is Already Known?
• Drotaverine is useful for providing relief
from pain in spastic and motility disorders of smooth muscles in
adults.
What Thid Study Adds?
• Drotaverine provides symptomatic relief in children with
recurrent abdominal pain.
|
References
1. Apley J, Naish N. Recurrent abdominal pains: A
field survey of 1000 school children. Arch Dis Child.
1958;33:165-70.
2. Faull C, Nicol AR. Abdominal pain in
six-year-olds: An epidemiological study in a new town. J Child Psychol
Psychiatry Allied Disciplines. 1986;27:251-60.
3. Paul SP, Bannasrd P, Bigwood C, Candy DC.
Challenges in management of irritable bowel syndrome in children. Indian
Pediatr. 2013;50:1137-43.
4. Abu-Arafeh I, Russell G. Prevalence and clinical
features of abdominal migraine compared with those of migraine headache.
Arch Dis Child. 1995;72:413-7.
5. Mayer EA, Tillisch K. The brain-gut axis in
abdominal pain syndromes. Annu Rev Med. 2011;62:381-96.
6. Huertas-Ceballos AA, Logan S, Bennett C, Macarthur
C. Pharmacological interventions for recurrent abdominal pain (RAP) and
irritable bowel syndrome (IBS) in childhood. Cochrane Database of
Systematic Reviews 2008;1:CD003017.
7. Illingworth RS. Evening colic in infants. A
double-blind trial of dicyclomine hydrochloride. Lancet. 1959;2:
1119-20.
8. Grunseit F. Evaluation of the efficacy of
dicyclomine hydrochloride (Merbentyl) syrup in the treatment of infant
colic. Curr Med Res Opin. 1977;5:258-61.
9. Weissbluth M, Christoffel KK, Davis AT. Treatment
of infantile colic with dicyclomine hydrochloride. J Pediatr.
1984;104:951-5.
10. European Medicines Agency. Report on the Survey
of all Paediatric Uses of Medicinal Products in Europe. EMA/794083/2009.
Available from:
www.ema.europa.eu/docs/en_GB/document_library/Report/2011/01/wc500101006.
pdf. Accessed July 14, 2015.
11. McGrath PA, Seifert CE, Speechley KN, Booth JC,
Stitt L, Gibson MC. A new analogue scale for assessing children’s pain:
an initial validation study. Pain. 1996;64:435-43.
12. von Baeyer CL. Children’s self-reports of pain
intensity: Scale selection, limitations and interpretation. Pain Res
Manag. 2006;11:157-62.
13. Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E,
Hyams JS, Staiano A, et al. Childhood functional gastrointestinal
disorders: child/adolescent. Gastroenterology. 2006; 130:1527-37.
14. Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher
DR, Hyams JS, Milla PJ, et al. Childhood functional
gastrointestinal disorders. Gut. 1999;45:60-8.
15. Kline RM, Kline JJ, Di Palma J, Barbero GJ.
Enteric-coated, pH dependent peppermint oil capsules for the treatment
of irritable bowel syndrome in children. J Pediatr. 2001;138:125-8.
16. Symon DN, Russell G. Double blind placebo
controlled trial of pizotifen syrup in the treatment of abdominal
migraine. Arch Dis Child. 1995;72:48-50.
17. Sanders MR, Rebgetz M, Morrison M, Bor W, Gordon
A, Dadds M, et al. Cognitive-behavioral treatment of recurrent
nonspecific abdominal pain in children: an analysis of generalization,
maintenance, and side effects. J Consult Clin Psychol. 1989;57:294-300.
18. Sanders MR, Shepherd RW, Cleghorn G, Woolford H.
The treatment of recurrent abdominal pain in children: a controlled
comparison of cognitive-behavioral family intervention and standard
pediatric care. J Consult Clin Psychol. 1994;62:306-14.
19. See MC, Birnbaum AH, Schechter CB, Goldenberg MM,
Benkov KJ. Double-blind, placebo-controlled trial of famotidine in
children with abdominal pain and dyspepsia: global and quantitative
assessment. Dig Dis Sci. 2001;46:985-92.
20. Feldman W, McGrath P, Hodgson C, Ritter H,
Shipman RT. The use of dietary fiber in the management of simple,
childhood, idiopathic, recurrent, abdominal pain. Results in a
prospective, double-blind, randomized, controlled trial. Am J Dis Child.
1985;139:1216-8.
21. Lebenthal E, Rossi TM, Nord KS, Branski D.
Recurrent abdominal pain and lactose absorption in children. Pediatrics.
1981;67:828-32.
22. Dash A, Maiti R, Akantappa Bandakkanavar TK,
Arora P. Intramuscular drotaverine and diclofenac in acute renal colic:
A comparative study of analgesic efficacy and safety. Pain Med.
2012;13:466-71.
23. Romics I, Molnár DL, Timberg G, Mrklic B,
Jelakovic B, Köszegi G, et al. The effect of drotaverine
hydrochloride in acute colicky pain caused by renal and ureteric stones.
BJU Int. 2003;92:92-6.
24. Rai RR, Dwivedi M, Kumar N. Efficacy and safety
of drotaverine hydrochloride in irritable bowel syndrome: A randomized
double-blind placebo-controlled study. Saudi J Gastroenterol.
2014;20:378-82.
|
|
|
 |
|

