Japanese encephalitis (JE) is one of the commonest
causes of acute encephalitis syndrome (AES) in many states of India.
According to the Directorate of National Vector Borne Disease Control
Programme (NVBDCP), Delhi, 1661 cases of JE were reported in the year
2014 from 15 states and union territories, out of which 293 (17.6%) died
[1]. Assam, West Bengal, Uttar Pradesh (UP) and Jharkhand reported
maximum number of cases.
JE vaccination in India started in 2006 following
large outbreaks of JE in some districts of Eastern UP and Bihar. Large
vaccination campaigns were carried out in 11 of the highest risk
districts of the country in 2006, 27 districts in 2007, 22 districts in
2008, and 30 districts in 2009. Children between the age group of 1 to
15 years were vaccinated with a single dose of Chinese live attenuated
SA-14-14-2 JE vaccine [2].In 2011, the same SA-14-14-2 JE vaccine was
introduced in the routine immunization under Universal Immunization
Program (UIP) in the 181 endemic districts as a single dose at 16 to 18
months at the time of 1st booster of DTP vaccine. In 2013, another dose
of SA-14-14-2 vaccine was added at 9 months of age along with measles
vaccine [3]. So far, 155 out of 181 identified JE endemic districts are
covered under JE campaign and overall 10.8 crore children have been
immunized with JE vaccine through campaigns [3].
Following mass vaccination campaigns with live
attenuated SA-14-14-2 JE vaccine among pediatric age group, adult JE
cases have outnumbered pediatric cases in some JE endemic states,
including Assam. This led the state government of Assam to conduct
special campaigns of JE vaccines in adults (>15 years) in some most
affected districts [2]. The exact reason behind this shift in age group
is not well understood.
On 3rd July, 2014 the Government of India (GOI)
announced the introduction of four new vaccines, including JE vaccine,
in the National immunization program. The JE vaccine would be available
for adults in 179 districts in nine states where the disease is highly
prevalent [4].
Recently, NVBDCP has identified 20 high burden
districts in three states–Assam [5], Uttar Pradesh [7], and West Bengal
[8], for adult JE vaccination (>15-65 years). Till now, eight districts
have been covered by the adult vaccination programme [5].
Adult JE Vaccination Program: Is it Prudent ?
The policy to immunize adults in JE endemic areas is
fraught with imponderables, and may not be wise economically. It would
be desirable if the following are factored before putting into operation
this exercise which may not achieve the intended objective:
• Is mass vaccination of children responsible for
age-shift of the disease toward adults?
• Is adult vaccination the only option for
controlling adult JE?
• Can adult immunization be carried out
independently of childhood vaccination?
• Will this exercise in adults give durable
immunity without the need for periodic boosters?
• Are there adequate research data available to
justify this costly exercise?
JE mostly affects children. Majority of adults in
endemic areas have developed immunity to JE due to sub-clinical
infection or clinical infection during childhood. Why should a vaccine
be administered to an immune adult who is unlikely to suffer clinical
illness on exposure? Few adults affected during outbreaks in endemic
areas are either non-immune or live in areas of new invasion by the
virus, or are infected by a variant virus. Vaccination in such instances
is purposeless. As per the government declaration, the main target for
the vaccine is "endemic areas" and not "emerging areas" of JE disease.
Even assuming that more number of adults suffer disease in endemic
areas, the implication is that the natural immunity is ineffective or
the infecting JE strain is a variant, and the efficacy of vaccine in
this situation is questionable.
Further, the ‘vaccine-take’ in childhood is much
better than in adults. Given the fact that majority of adults are
immune, the vaccination program aiming at protecting the minuscule
non-immune residual adults must achieve 100% immunization coverage, an
unrealistic task in the Indian context. Furthermore, even a single dose
of vaccine may not seroconvert all the seronegative adults. A subset
would still remain seronegative and susceptible to infection and
disease. Valuable resources should be better utilized by focusing on
disease prevention in children, the main group afflicted by the disease.
The integrated vector-control measures should be prioritized over the
move to immunize adult population.
Continuing JE Vaccination Program: Is it Justified?
JE only represents 14-15% of all AES cases in the
country [1]. Many non-infectious, non-encephalitic illnesses like
encephalopathy are included in the broad group of AES. Even,
enteroviruses are coming in a big way as far as the encephalitis group
of illnesses covered in AES is concerned [9]. At the same time,
non-availability of diagnostic facilities for JE at district level has
severely hampered the quality of AES surveillance in the country. It is
now debatable to continue a national program to control a highly
localized illness with around 1000 cases and 200-odd deaths every year
[1]. As the disease almost exclusively affects the rural residents,
vaccination of individuals residing in urban areas seems redundant.
Since humans are not the only reservoirs of the virus, it is highly
improbable to eliminate JE infection from the community. On the other
hand, many experts are concerned at the continued neglect of some more
serious, significant public health problems like rabies – a universally
prevalent entity killing around 20,000 people every year in the country
[6].
Efficacy and Effectiveness of the JE Vaccine in India
There are issues pertaining to effectiveness of
currently employed SA-14-14-2 JE vaccine in India. Despite using this
vaccine in campaigns and later in Routine immunization, there is no
appreciable change in epidemiology of JE in India (Fig. 1).
There are contradictory reports regarding efficacy and effectiveness of
this vaccine in India.
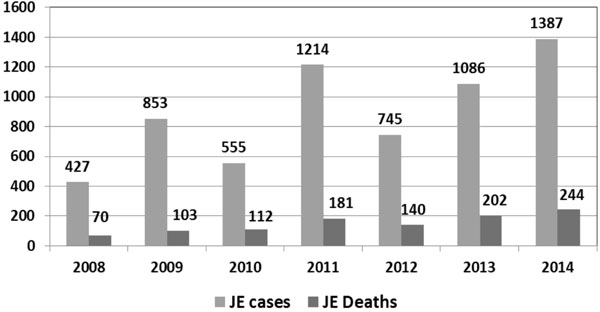 |
|
Fig. 1 Number of cases and deaths due
to JE in India (Source: Directorate of National Vector
Borne Disease Control Programme;
http://nvbdcp.gov.in/Doc/je-aes-cd-May15.pdf.)
|
In the neighboring country Nepal, the protective
efficacy of a single dose of SA-14-14-2 JE vaccine was found as high as
98.5% (CI: 90.1-99.2%) 12-15 months after administration [7]. A small
case-control study from Lucknow, India found an efficacy of 94.5% after
a single dose of this vaccine within 6 months after its administration
[8]. An unmatched case-control study among children aged 24-54 months
from Gorakhpur division in India found 84% effectiveness of this vaccine
despite a low coverage 51% [9].
However, a post-marketing surveillance (PMS) in India
conducted by ICMR revealed that the efficacy of the vaccine in India was
not as high as that seen in Nepal. This study showed that virus
neutralizing antibodies were seen in 45.7% of children before
vaccination. Sero-conversion against Indian strains 28 days after
vaccination was 73.9% and 67.2% in all individuals and in those who were
non-immune pre-vaccination, respectively. The protective efficacy of the
vaccine at one year was 43.1% overall, and 35% for those who were
non-immune pre-vaccination [10].
Preliminary results of another case-control study
carried out by ICMR on the impact of JE vaccine shows an unadjusted
protective effect of 62.5% in those with any report of vaccination [10].
According to this report, the JE vaccine efficacy has been calculated at
60% in UP, and around 70% in Assam. Following this report, ICMR has
recommended a study on the impact of 2 doses vs. single dose of
SA-14-14-2 vaccine in Assam [10].
Thus there is no conclusive data on the precise
efficacy/effectiveness of currently employed JE vaccine in India. Few
more antigens from indigenous producers are now available; a thorough
reappraisal of the policy to use only Chinese product in the program is
urgently warranted.
Conclusions
There is an urgent need of reappraisal of the policy
of mass JE vaccination in the country. The quality of surveillance needs
bolstering with availability of diagnostic facility at district health
centers. There should be more targeted use of available JE vaccines in
affected areas. There is an urgent need to collect precise effectiveness
data of the currently employed Chinese JE vaccine in the program. It
would be ideal to explore the possibility of employing newer antigens
after proper cost-effectiveness exercises. The decision to mass
vaccinate adults against JE in the entire district should be reviewed
again.
In the end, the axiom, "prevention is likely to
override other measures in maintaining healthy nation" should not lose
its primacy, and efforts in this direction, including integrated vector
control measures must be stepped up. Public health efforts should not
focus on vaccination alone.
1. Directorate of National Vector Borne Disease
Control Program- Delhi. Details of AES/JE Cases and Deaths from
2008-2014. Available from:
http://nvbdcp.gov.in/Doc/je-aes-cd-May15.pdf. Accessed June 15,
2015.
2. Japanese encephalitis vaccines. In:
Vashishtha VM, Choudhury P, Bansal CP, Yewale VN, Agarwal R. editors.
IAP Guidebook on Immunization 2013-2014. National Publication House,
Indian Academy of Pediatrics, Gwalior, 2014.
3. Universal Immunization Program, Immunization
Division at MoHFW. Available from:http://www.nhp.gov.in/sites/default/files/pdf/immunization_uip.pdf.
Accessed June 16, 2015.
4. Datta J. Four vaccines added to India’s
immunisation programme. Available from: http://www.thehindubusiness
line.com/economy/policy/four-vaccines-added-to-indias-immunisation-programme/article6173880.ece.
Accessed June 18, 2015.
5. Anonymous. State begins JE vaccination drive.
Available from: http://timesofindia.indiatimes.com/city/guwahati/State-begins-JE-vaccination-drive/articleshow/46707173
Cms. Accessed June 18, 2015.
6. Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS,
Ashwath Narayana DH, Abdul Rahman S, et al. Assessing the burden
of human rabies in India: Results of a national multi-center
epidemiological survey. Int J Infect Dis. 2007;11:29-35.
7. Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP,
Halstead SB. Effect of a single dose of SA-14-14-2 vaccine one year
after immunization in Nepalese children with Japanese Encephalitis: A
case control study. Lancet. 2005;366:1375-8.
8. Kumar R, Tripathi P, Rizvi A. Effectiveness of one
dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med.
2009;360:1465-6.
9. Murhekar MV, Ranjan P, Selvaraju S, Pandey A, Gore
MM, Mehendale SM. Low coverage and acceptable effectiveness of single
dose of Japanese encephalitis vaccine, Gorakhpur division, Uttar
Pradesh, India, 2013. J Infect. 2014;69:517-20.

