|
|
|
Indian Pediatr 2014;51: 811-817 |
 |
Is Two Month Initial Prednisolone Treatment
for Nephrotic Syndrome
Inferior to Longer Duration Therapy?
|
|
Source Citation: Yoshikawa N, Nakanishi K, Sako M, Oba
MS, Mori R, Ota E, et al., for the Japanese Study Group of Kidney
Disease in Children. A Multicentre Randomized Trial Indicates Initial
Prednisolone Treatment for Childhood Nephrotic Syndrome for Two Months
is not Inferior to Six-month Treatment. Kidney Int. 2014;
doi:10.1038/ki.2014.260
Section Editor: Abhijeet Saha
|
|
Summary
This multi-center randomized trial [1] in children
with steroid sensitive nephrotic syndrome (NS) compared prednisolone
therapy for 2 months versus 6 months. Authors evaluated time to
occurrence of frequent relapse, time to first relapse, and various
adverse events in 255 children (1-15 years) from 90 Japanese hospitals
presenting with first episode of NS. Those who achieved remission within
3 weeks of prednisolone therapy were randomized to either 6 months
therapy [60 mg/m 2 daily for
4 wk, gradually (4 weekly) tapering alternate day doses for next 24 wk]
or 2 months therapy (60 mg/m2
daily for 4 wk, 40 mg/m2
alternate day for 4 wk) with oral prednisolone. Children were followed
up for at least 2 years. The authors reported comparable (i) time to
frequent relapses (RR 0.86, 95% CI 0.64, 1.16), (ii) episodes of relapse
(RR 0.92, 95% CI 0.75, 1.14), (iii) frequent relapses (RR 0.99, 95% CI
0.72, 1.38), (iv) time to first relapse, (v) relapse rate per
person-year, and (vi) adverse effects (hypertension, cushingoid facies,
glaucoma, and elevated hepatic enzymes). The authors also presented a
meta-analysis comparing trials using 2-3 versus 5-6 months
therapy, and reported comparable results. They suggested that 2 months
of prednisolone is not inferior to 6 months therapy.
Commentaries
Contemporary Researchers’ Viewpoint
Most patients with steroid sensitive nephrotic
syndrome show highly satisfactory renal outcomes [2]. While morbidity
due to infections has declined with rapid diagnosis and use of vaccines,
toxicities associated with repeated course of corticosteroids remain a
major concern in managing patients with frequent relapses or steroid
dependence. While the International Study for Kidney Diseases in
Children (ISKDC) arbitrarily proposed that the initial corticosteroid
therapy comprise of 4-weeks daily followed by 4-weeks intermittent
therapy, refinements have been proposed over the last four decades. A
randomized controlled trial in 1993 showed reduced relapse rates on
prolonging therapy from 8 to 12 weeks [3]. Further randomized studies
confirmed these findings and suggested that extending initial therapy to
6-months was even better. Results from a Cochrane meta-analysis showed
that therapy for 6-months versus 3-months was associated with
reduced risk of frequent relapses (RR 0.55; 95% CI 0.39,0.80) and fewer
relapses per year. The review suggested an inverse relationship between
the risk for relapse and duration of induction therapy, such that the
relative risk for relapse at 12-24 months would fall by 11% of baseline
relapse rate for every one month increase in duration of therapy from
2-7 months [4]. While studies included in the analysis had
methodological concerns, the results unequivocally suggested that (i)
12-weeks therapy was better than 8-weeks, and (ii) therapy could
be extended to 6-months with further benefits in rates of sustained
remission and reduced frequency of relapses.
Three recent well designed randomized studies contest
the above view. A multicenter placebo-controlled parallel group trial
from Netherlands, on 150 children, showed no differences in the
cumulative proportion of children with frequent relapses (45 versus
50%) or any relapse (77% versus 80%) when initial therapy was
prolonged from 12 to 24 weeks without increasing the cumulative dose
[5]. Similarly, a randomized placebo-controlled blinded trial conducted
across 5 centers in North India on 181 patients randomized to receive 6
or 3 months of prednisolone differing in cumulative dose by 739 mg/m 2,
showed no differences in the frequency of relapses at one year, hazard
for frequent relapses, and proportions with sustained remission or
steroid adverse effects [6]. Post-hoc subgroup analysis showed
that children younger than 3 year might benefit from 6 months therapy
with reduced risk for first relapse, but not for frequent relapses,
suggesting that prolonged therapy requires closer evaluation in this
subgroup. The open label multicenter randomized trial from Japan,
published simultaneously with the Indian study, examined the
non-inferiority of 2 months to 6 months therapy with prednisone at
higher cumulative dose (2240 versus 3840 mg/m2)
in 255 patients, 1-15 yr-old, followed for 2 years [7]. The definition
for relapse used in this study, 2+ or more proteinuria, was liberal than
that used otherwise, which may have led to overestimating the rates of
relapses and frequent relapsers. Based on these results, the authors
conclude that initial steroid therapy for two months, despite less
medication exposure, was not inferior to 6 months treatment in affecting
the rates of frequent relapses.
Results from these three studies that enrolled almost
600 patients on two continents emphasize that prolongation of initial
therapy to 6 months is not useful in modifying the course of the
disease, or reducing subsequent needs for corticosteroids and
steroid-sparing agents. The Indian study, but not the others, showed
that the benefit of extended initial therapy was limited to the period
while the steroids were being administered [1,5,6]. Since the intent of
intensification of therapy is to alter the disease course rather than
delaying the first relapse, lower rates of relapses during steroid
tapering alone should not lead to consideration of prolonged therapy.
Furthermore, the benefits of therapy prolongation should be balanced
against the risk of corticosteroid adverse effects. Given the current
data, prolongation of initial therapy beyond that proposed by the ISKDC
[2] and the APN [4] is perhaps not justified. Recommendations of the
Indian Society of Pediatric Nephrology [7], Kidney Diseases Improving
Global Outcomes [8] and the Canadian Society of Pediatric Nephrology [9]
are likely to remain unchanged in endorsing 12 weeks therapy for the
initial episode of nephrotic syndrome.
Aditi Sinha and Arvind Bagga
Department of Pediatrics
AIIMS, New Delhi, India.
Email:
[email protected]
Pediatric Nephrologist’s Viewpoint
This multicentric open-labeled non-inferiority trial,
undertaken in Japan, randomized 255 children with the first episode of
nephrotic syndrome, to receive either 2 month or 6 month therapy with
oral prednisolone. The end points of the initial course of therapy were
similar in both groups, with a lower mean cumulative dose of steroids in
the 2 month therapy group compared to the 6 month therapy arm. In the
past decade, as evident in the Cochrane reviews, children with first
episode of nephrotic syndrome, treated with prednisone for at least
three months resulted in fewer relapse rates by 12 to 24 months with an
increase in benefit being demonstrated for up to seven months of
treatment compared to two months therapy (ISKDC regime) [4]. In contrast
to this finding, certain studies [5,10]
failed to show benefit of prolonged duration of
prednisolone in reducing the frequency of relapses, despite maintaining
equal cumulative dose of steroids between groups in the Dutch study [5].
Recently, a well-designed, placebo controlled randomized trial in Indian
children revealed that
extending initial prednisolone treatment from 3 to 6 months is not
effective in modifying the course of disease and reducing subsequent
need for corticosteroids, within a year of follow up, in children with
nephrotic syndrome [6].
In clinical practice, the major challenge in treating
nephrotic syndrome is to reduce the rate of relapses and minimize the
adverse effects of steroid therapy. While there seems to be new evidence
that supports the hypothesis that initial duration of steroid therapy
has limited impact on relapse rates, a few essential points need to be
considered at this juncture. First, the interpretation of relapses in
most studies is based on follow up period of 12-24 months, which is
relatively a short time span compared to the well-known highly variable
clinical course of nephrotic syndrome that lasts for more than a decade.
The impact of initial cumulative dose of steroid therapy, besides the
duration, needs further evaluation. Second, it would be essential to
consider the baseline rate of relapse in a particular community, as most
relapses are triggered by infections that may be independent of the
duration or dose of initial steroid therapy. Third, we need clarity on
the precise effect of age of the child at onset of nephrotic syndrome,
on the occurrence of relapses. To conclude, though we may not be ready
with a recommendation, we are encouraged to be conscious of the fact
that intentional prolongation of initial steroid therapy, though may not
predispose to higher steroid toxicity, may not be as beneficial as we
believed it to be, in reducing relapse rates in nephrotic syndrome.
Arpana Iyengar
Department of Pediatric Nephrology,
St John’s Medical College
Bangalore, India
Email:
[email protected]
Evidence-based-medicine Viewpoint and Systematic
Review
Relevance : Some
decades back, ISKDC advocated an eight-week steroid regimen for
induction and maintenance of remission in children with nephrotic
syndrome (NS) [11]. This was followed for several years, whereupon it
was observed that while remission was achieved in most children, the
majority relapsed and a quarter to half develop frequent relapses
[2,12,13]. Therefore, trials comparing longer (≥
3 mo) therapy with the then-standard two months regimen were undertaken.
These trials and subsequent systematic reviews including a Cochrane
review last updated in 2007 [4] suggested that longer duration of
therapy was associated with better outcomes. The relatively low
methodological quality of trials on which this conclusion was based, and
the natural concern about adverse effects of therapy with longer steroid
regimen prompted some investigators to revisit the issue. An additional
confounding factor is that longer duration of therapy is associated with
higher total dosage of steroid; therefore it is difficult to determine
whether longer duration or higher dose or both together account for
better outcomes with such regimens. The outdated Cochrane review [4,14]
argued in favor of increased duration (rather than higher dose). There
is uncertainty about the optimal steroid regimen that could achieve the
triple goal of inducing remission, preventing relapses, and ensuring
safety in terms of avoidance of steroid-related adverse events. Against
this backdrop, the trial by Yoshikawa, et al. [1] is very timely
and relevant.
Critical appraisal: Two angles need
exploration viz (i) appraisal of the trial itself, and (ii)
contextualizing the evidence from this trial, in terms of the clinical
question viz what is the optimal steroid regimen in children with
NS?
In terms of methodological quality, the trial
utilized an adequate method for generation of randomization sequence.
Allocation concealment was not described and blinding was absent. Of 255
enrolled children, the authors could report outcomes in 246 with
comparable loss in both groups. Unlike most such trials, the authors
chose a non-inferiority design with its associated (appropriate)
calculation of sample size. They chose risk ratio of time to frequently
relapsing NS (FRNS) as the primary outcome, rather than the more
commonly presented frequency of relapses or FRNS. Standard definitions
were used for the various conditions. Steroid adverse effects were
comparable between the groups, generally occurred early, and were
transient. The authors concluded that this indicates that the shorter
duration could be recommended since it delivers a lower total dose.
However, this conclusion is not supported by the data in the trial. The
meta-analysis presented as a Supplement contains some methodological
errors (incorrect data entry), hence need not be considered.
This necessitates a fresh look at the available
evidence. As 3 months therapy is the current recommended standard of
care [7-9,15], the following comparisons of efficacy are meaningful: (i)
3 months therapy versus >3 months; (ii) 3 months versus 2 months;
and (iii) 2 months versus >2 months. It is also important to
compare trials using the same dose (over different durations) as well as
same duration (with different doses). Therefore, a fresh systematic
review was undertaken searching PubMed and the Cochrane Library. Two
sets of searches were conducted through PubMed using the terms: "(nephrotic
syndrome) AND steroid" with filters: Meta-Analysis, Systematic
Reviews, Randomized Controlled Trial, Child: birth-18 years; and
"(nephrotic syndrome) AND steroid AND duration" without any filters.
The Cochrane Library was searched using the term "nephrotic syndrome"
without any filters. Seventeen trials [1,12-27] were identified that
compared different durations and/or dose of prednisolone therapy and
evaluated relapse as an outcome over a period of at least 12 months
follow-up (Web Table I). One trial [27] comparing 12
months versus 5 months therapy was not included.
Summary of the meta-analyses of efficacy (risk ratio,
random effects model) is presented in Table I, showing
that therapy >3 months (with higher steroid dose) is more efficacious
than 3 months. Limited data suggest that longer duration or higher dose
alone did not make a difference. Therapy for 3 months was comparable to
2 months (with or without higher total dose). When therapy >2 months was
compared against 2 months, the former appeared more efficacious. These
findings are contrary to the results in Yoshikawa’s trial [1]. While the
overall results are in agreement with the outdated Cochrane review [4],
the assertion therein that longer duration rather than higher dose is
responsible for greater efficacy [4,15], could not be substantiated.
However, it should be remembered that the quality of individual trials
in the meta-analysis leaves a lot to be desired.
TABLE I Summary Of Meta-Analyses
|
Comparison |
Outcome |
Result
(RR, 95% CI) |
|
>3 mo (with higher dose) vs 3 mo therapy |
Relapse
|
0.58 (0.42,
0.79); 5 trials, 604 participants, I2=73% (Fig. 1A) |
|
Frequent relapses |
0.68 (0.54,
0.85); 5 trials, 604 participants, I2=45% (Fig. 1B) |
|
>3 mo vs 3 mo therapy (with same total |
Relapse |
1.03 (0.86,
1.24); 1 trial, 126 participants (Fig. 2A) |
|
dose in both groups) |
Frequent relapses |
1.11 (0.77,
1.60); 1 trial, 126 participants (Fig. 2B) |
|
Higher vs lower steroid dose (administered |
Relapse |
0.63 (0.42,
0.94); 1 trial, 59 participants (Fig. 3A) |
|
over same duration i.e 3 mo) |
Frequent relapses |
0.69 (0.35,
1.37); 1 trial, 60 participants (Fig. 3B) |
|
3 mo (with higher dose) vs 2 mo therapy |
Relapse |
0.91 (0.61,
1.38); 3 trials, 99 participants, I2=62% (Fig. 4A) |
|
Frequent relapses |
0.83 (0.55,
1.27); 3 trials, 99 participants, I2=2% (Fig. 4B) |
|
>3 mo (with higher dose) vs 2 mo therapy |
Relapse
|
0.71 (0.58,
0.88); 6 trials, 498 participants; I2=48% (Fig. 5A) |
|
Frequent relapses |
0.81 (0.65,
1.00); 6 trials, 742 participants, I2=40% (Fig. 5B) |
|
3 mo vs 2 mo therapy (with same total dose) |
Relapse |
1.09 (0.88,
1.36); 1 trial, 112 participants (Fig. 6A) |
|
Frequent relapses |
1.14 (0.78,
1.66); 1 trial, 112 participants (Fig. 6B) |
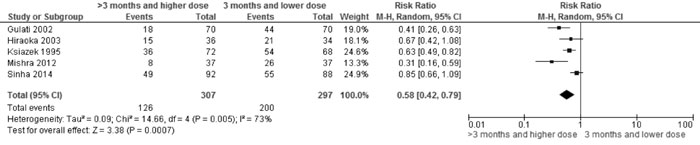
Fig 1A Comparison of >3 mo (with higher dose) vs 2 mo
therapy. Outcome: Relapse
|
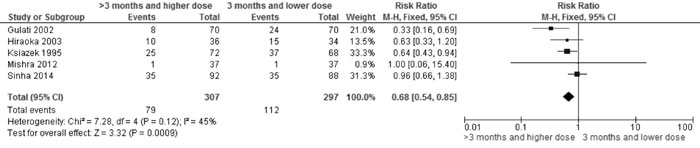
Fig 1B Comparison of >3 mo (with higher dose) vs 2 mo
therapy. Outcome: Frequent relapses
|
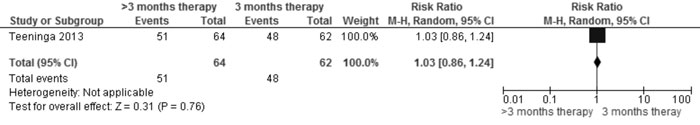
Fig 2A Comparison of >3 mo vs 3 mo therapy (with same
total dose in both groups). Outcome: Relapse
|
|
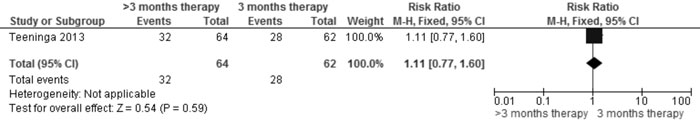

Fig 2B Comparison of >3 mo vs 3 mo therapy (with same
total dose in both groups). Outcome: Frequent relapses
|
|
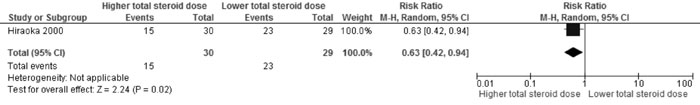
Fig 3A Comparison of Higher vs Lower steroid dose
(administered over same duration i.e 3 mo). Outcome: Relapse
|
|
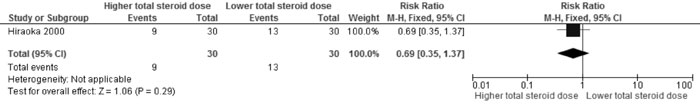
Fig 3B Comparison of Higher vs Lower steroid dose
(administered over same duration i.e 3 mo). Outcome: Frequent
relapses
|
|
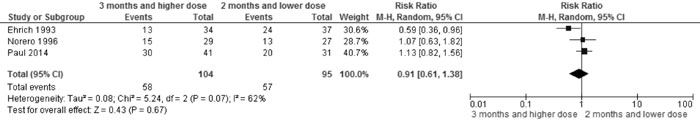
Fig 4A Comparison of 3 mo (with higher dose) vs 2 mo
therapy. Outcome: Relapse
|
|
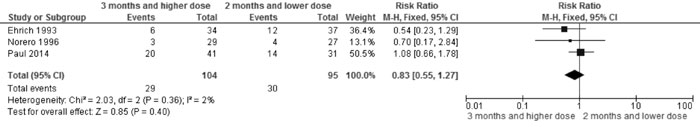
Fig 4B Comparison of 3 mo (with higher dose) vs 2 mo
therapy. Outcome: Frequent relapses
|
|
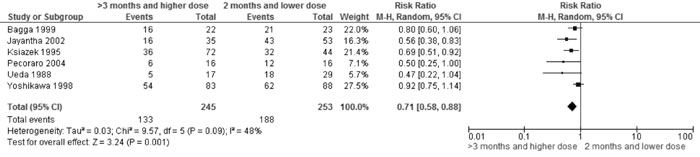
Fig 5A Comparison of >3 mo (with higher dose) vs 2 mo
therapy. Outcome: Relapse
|
|
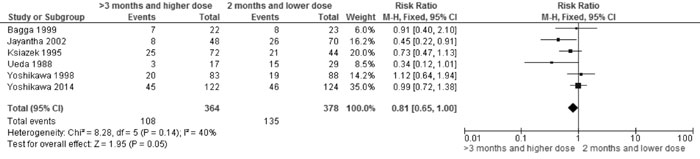
Fig 5B Comparison of>3 mo (with higher dose) vs 2 mo
therapy. Outcome: Frequent relapses
|
|
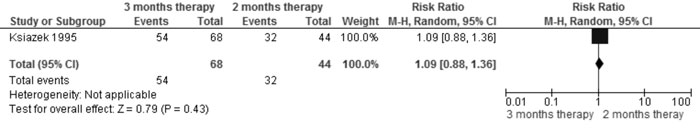
Fig 6A Comparison of 3 mo vs 2 mo therapy (with same
total dose). Outcome: Relapse
|
|
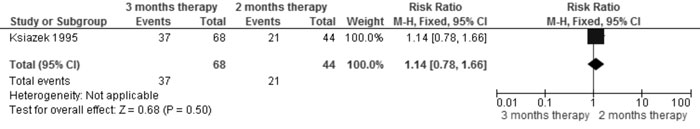
Fig 6B Comparison of 3 mo vs 2 mo therapy (with same
total dose). Outcome: Frequent relapses
|
It is also important to consider safety issues. The
diverse sets of data made meta-analysis difficult. However, almost all
the trials showed comparable frequency of adverse events. More
importantly, most adverse events occurred during the initial (more
intensive) phase of therapy, wherein the dosage and duration are more
comparable. An indirect point to note is that the lower efficacy of
shorter duration/lower total dose may be associated with more relapses
and hence more doses of prednisolone, thereby increasing the potential
for adverse events.
Extendibility: The issues of optimal
management of childhood nephrotic syndrome and the ideal steroid regimen
are relevant to the Indian setting. Although the results of the trial
cannot be extended to our setting, the findings of the new systematic
review presented here are applicable.
Conclusions: Although the trial suggests
that 2 months steroid therapy may be non-inferior to 6 months therapy,
the overall conclusion based on evaluation of all available data
(through a fresh systematic review) suggests that prednisolone therapy
longer than 3 months (with higher dose) is more efficacious than that
for 3 months or less.
Joseph L Mathew
Department of Pediatrics,
PGIMER, Chandigarh, India.
Email:
[email protected]
References
1. Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R,
Ota E, et al., for the Japanese Study Group of Kidney Disease in
Children. A multicenter randomized trial indicates initial
prednisolone treatment for childhood nephrotic syndrome for two months
is not inferior to six-month treatment. Kidney Int. 2014;doi
10.1038/ki.2014.260.
2. Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr.
Prognostic significance of the early course of minimal change
nephrotic syndrome: Report of the International Study of Kidney Disease
in Children. J Am Soc Nephrol. 1997;8:769-76.
3. Ehrich JH, Brodehl J. Long versus standard
prednisone therapy for initial treatment of idiopathic nephrotic
syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie.
Eur J Pediatr. 1993;152:357-61.
4. Hodson EM, Willis NS, Craig JC. Corticosteroid
therapy for nephrotic syndrome in children. Cochrane Database Syst Rev.
2007;4:CD001533.
5. Teeninga N, Kist-van Holthe J, van Rijskwijk N,
Hop WC, Wetzels JF, et al. Extending prednisolone therapy does
not reduce relapse in childhood nephrotic syndrome. J Am Soc Nephrol.
2012;24:149-59.
6. Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta
A, et al. Extending initial prednisolone treatment in a
randomized control trial from 3- to 6-months did not significantly
influence the course of illness in children with steroid sensitive
nephrotic syndrome. Kidney Int. 2014;doi: 10.1038/ki.2014.240.
7. Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD,
Senguttuvan P, et al. Indian Pediatric Nephrology Group, Indian
Academy of Pediatrics. Management of steroid sensitive nephrotic
syndrome: Revised guidelines. Indian Pediatr. 2008;45:203-14.
8. Kidney Disease Improving Global Outcomes (KDIGO)
Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for
Glomerulonephritis. Kidney Int. 2012; Suppl 2:139-274.
9. Samuel S, Bitzan M, Zappitelli M, Dart A, Mammen
C, Pinsk M, et al. Canadian Society of Nephrology Commentary on
the 2012 KDIGO clinical practice guideline for glomerulonephritis:
Management of nephrotic syndrome in children. Am J Kidney Dis.
2014;63:354-62.
10. Bagga A, Hari P, Srivastava RN. Prolonged versus
standard prednisolone therapy for initial episode of nephrotic syndrome.
Pediatr Nephrol. 1999;13:824-27.
11. Arneil GC. The nephrotic syndrome. Pediatr Clin
North Am. 1971;18:547-59.
12. Koskimies O, Vilska J, Rapola J. Long-term
outcome of primary nephrotic syndrome. Arch Dis Child 1982;57:544-8.
13. Nakanihi K, Iijima K, Ishikura K, Hataya H,
Sasaki S, Honda M, et al. Two-year outcome of the ISKDC regimen
and frequent-relapsing risk in children with idiopathic nephrotic
syndrome. Clin J Am Soc Nephrol. 2013;8: 756-62.
14. Hodson EM, Craig JC. Corticosteroid therapy for
steroid-sensitive nephrotic syndrome in children: Dose or duration? J Am
Soc Nephrol. 2013;24:7-9.
15. Bagga A, Indian Pediatric Nephrology Group,
Indian Academy of Pediatrics. Revised guidelines for management of
steroid-sensitive nephrotic syndrome. Indian J Nephrol 2008;18:31-9.
16. Gulati S, Ahmed M, Sharma RK, Gupta A, Pokhariyal
S. Comparison of abrupt withdrawal versus slow tapering regimen of
prednisolone therapy in the management of first episode of steroid
responsive childhood idiopathic nephrotic syndrome [abstract]. Nephrol
Dial Transplant. 2001;16:A87.
17. Hiraoka M, Tsukahara H, Haruki S, Hayashi S,
Takeda N, Miyagawa K, et al. Older boys benefit from higher
initial prednisolone therapy for nephrotic syndrome. The West Japan
Cooperative Study of Kidney Disease in Children. Kidney Int. 2000;
58:1247-52.
18. Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M,
Takeda N, Haruki S, et al. A randomized study of two long course
prednisolone regimens for nephrotic syndrome in children. Am J Kidney
Dis. 2003;41:1155-62.
19. Jayantha UK. Comparison of ISKDC regime with a
six month steroid regime in the treatment of steroid sensitive nephrotic
syndrome [abstract]. 7th Asian Congress of Pediatric Nephrology; 2000
Nov 1-4; Singapore. 2000.
20. Ksiazek J, Wyszynska T. Short versus long initial
prednisone treatment in steroid-sensitive nephrotic syndrome in
children. Acta Paediatr. 1995;84:889-93.
21. Mishra OP, Thakur N, Mishra RN, Prasad R.
Prolonged versus standard prednisolone therapy for initial episode of
idiopathic nephrotic syndrome. J Nephrol. 2012; 25:394-400.
22. Norero C, Delucchi A, Lagos E, Rosati P. Initial
therapy of primary nephrotic syndrome in children: Evaluation in a
period of 18 months of two prednisone treatment schedules. Chilean
Co-operative Group of Study of Nephrotic Syndrome in Children. Revista
Medica de Chile. 1996;124:567-72.
23. Paul SK, Muinuddin G, Jahan S, Begum A, Rahman
MH, Hossain MM. Long versus standard initial prednisolone therapy in
children with idiopathic nephrotic syndrome. Mymensingh Med J.
2014;23:261-7.
24. Pecoraro C, Caropreso MR, Malgieri G, Ferretti
AVS, Raddi G, Piscitelli A, et al. Therapy of first episode of
steroid responsive nephrotic syndrome: A randomised controlled trial.
Nephrol Dial Transplant. 1982;3:45.
25. Ueda N, Chihara M, Kawaguchi S, Niimomi Y, Nonada
T, Matsumoto J, et al. Intermittent versus long-term tapering
prednisolone for initial therapy in children with idiopathic nephrotic
syndrome. J Pediatr. 1988;112:122-6.
26. Yoshikawa N, Ito H, Takehoshi Y, Honda M, Awazu
M, Iijima K, et al. Standard versus long-term prednisolone with
Sairei-to in childhood steroid-responsive nephrotic synd-rome: a
prospective controlled study. Nippon Jinzon Gakkai Shi. Japanese J
Nephrol. 1998;40:587-90.
27. Kleinknecht C, Broyer M, Parchoux B, Loriat C,
Nivet H, Palcoux JB, et al. Comparison of short and long
treatment at onset of steroid sensitive nephrosis (SSN). Preliminary
results of a multicenter controlled trial for the French Society of
Pediatric Nephrology.
|
|
|
 |
|

