|
|
|
Indian Pediatr 2021;58:1059-1066 |
 |
Factors Associated With Neonatal Pneumonia
and its Mortality in India: A Systematic Review and
Meta-Analysis
|
|
N Sreekumaran Nair, 1
Leslie Edward Lewis,2 Vijay
Shree Dhyani,1 Shruti
Murthy,1 Myron Godinho,1
Theophilus Lakiang,1 Bhumika
T Venkatesh1
From 1Department of Statistics, Public Health Evidence South Asia
(PHESA); and 2Department of Pediatrics, Kasturba Medical College
Hospital; Manipal Academy of Higher Education, Manipal, Karnataka.
Correspondence to: Dr Bhumika T Venkatesh, Room no. 35,
Public Health Evidence South Asia (PHESA), Prasanna School of Public
Health, Manipal Academy of Higher Education, Madhav Nagar, Manipal,
Karnataka.
Email:
[email protected]
Received: May 19, 2020;
Initial review: June 29, 2020;
Accepted: March 13, 2021.
Protocol registration: PROSPERO 2016 CRD42016044019 (risk
factors);
PROSPERO 2016 CRD42016045398 (mortality)
|
Background: Neonatal pneumonia remains a significant contributor to
infant mortality in India and responsible for increased prevalence of
infant deaths globally. Objective: To identify risk factors
associated with neonatal pneumonia and its mortality in India. Study
design: A systematic review was conducted including both
analytic study designs and descriptive study designs, which reported a
quantitative analysis of factors associated with all the three types of
pneumonia among neonates. The search was conducted from August to
December, 2016 on the following databases; CINAHL, EMBASE, Ovid MEDLINE,
PubMed, ProQuest, SCOPUS, Web of Science, WHO IMSEAR and IndMED. The
search was restricted to Indian setting. Participants: The
population of interest was neonates. Outcomes: The outcome
measures included risk factors for incidences and mortality predictors
of neonatal pneumonia. These could be related to neonate, maternal and
pregnancy, caregiver, family, environment, healthcare system, iatrogenic
and others. Results: A total of three studies were
included. For risk factors, two studies on ventilator-associated
pneumonia were included with 194 neonates; whereas for mortality
predictors, only one study with 150 neonates diagnosed with pneumonia
was included. 11 risk factors were identified from two studies: duration
of mechanical ventilation, postnatal age, birth weight, prematurity, sex
of the neonate, length of stay in NICU, primary diagnosis, gestational
age, number of re-intubation, birth asphyxia, and use of nasogastric
tube. Meta-analysis with random-effects model was possible only for
prematurity (<37 week) and very low birth weight (<1500 g) and very low
birth weight was found to be significant (OR 5.61; 95% CI 1.76, 17.90).
A single study was included on predictors of mortality. Mean alveolar
arterial oxygen gradient (AaDO2) >250 mm Hg was found to be the single
most significant predictor of mortality due to pneumonia in neonates.
Conclusion: The study found scant evidence from India on risk
factors of neonatal pneumonia other than ventilator-associated
pneumonia.
Keywords: Alveolar-arterial oxygen gradient,
Respiratory distress, Risk factors, Ventilator-associated pneumonia.
|
|
N
eonatal pneumonia
accounts for 6.1% of total
global neonatal mortality whereas it contributes 5.1% to neonatal mortality in India and
5.6% in South Asia [1]. There is no international consensus
regarding definition, diagnostic criteria and management of
pneumonia among neonates [2, 3]. National nosocomial infections
surveillance (NNIS) 1996 and original Centers for Disease
Control (CDC) guidelines (pediatric modification) are commonly
followed for diagnosis of neonatal pneumonia.
It has been observed that poverty, limited
healthcare accessibility, and improper child-rearing practices
are some of the risk factors for pneumonia in young children
[4]. Other factors related to development of pneumonia,
particularly in India, are financial status, malnutrition, poor
immunization status, and household air pollution [5]. In South
East Asia, poor prenatal care, home delivery, fever at birth,
maternal urinary tract infections, prolonged rupture of membrane
were found as notable risk factors of neonatal pneumonia [6,7].
There is scanty information available on
neonatal pneumonia from India. Identification and elimination of
risk factors associated with neonatal pneumonia is imperative to
reduce its high prevalence and associated mortality, and
implementing appropriate interventions to improve neonatal
survival. With this review we intended to identify risk factors
and mortality predictors associated with neonatal pneumonia in
the Indian context.
METHODS
Protocol for these systematic reviews were
registered with PROSPERO [8,9] and published as separate
publications [10,11] where methodology is described in detail.
Ethical clearance was obtained from institutional ethics
committee of the host institution.
Studies reporting all types of neonatal
pneumonia published in English language in journals,
irrespective of peer reviewed or not were eligible for
inclusion. Studies on neonatal sepsis were also searched to
verify the presence or absence of a ‘pneumonia’ subgroup as
pneumonia is usually considered under the umbrella of neonatal
sepsis. To be eligible for inclusion, these articles had to
mention the outcomes specifically for neonatal pneumonia.
Both analytic study designs (case-control
studies, cohort studies, cross-sectional studies) and
descriptive study designs (case series, cross-sectional studies)
which report a quantitative analysis of factors associated with
all the three types of pneumonia among neonates were eligible
for inclusion. Letters, editorials, commentaries, reviews,
meta-analysis, qualitative research, conference papers and
reports were excluded.
Neonates diagnosed with any form of pneumonia
including community acquired pneumonia, congenital pneumonia and
hospital acquired pneumonia (ventilator associated pneumonia)
were included. The outcome measures included risk factors for
neonatal pneumonia and its mortality. These could be related to
neonate, maternal and pregnancy, caregiver, family, environment,
healthcare system, iatrogenic and others.
Search methods: Articles were
identified from nine databases (CINAHL, EMBASE, Ovid MEDLINE,
ProQuest, PubMed, SCOPUS, WHO IMSEAR, Web of Science and IndMED)
and government websites without time restriction. A separate
search was undertaken to identify risk factors and mortality
predictors associated with neonatal pneumonia. Detailed search
terms and strategy for PubMed for both the outcomes has been
provided in Web Appendix 1. The search on all the
databases was conducted from August to December, 2016. Some of
the search terms included were: "Risk factor" OR "determinant"
OR "risk" OR "predictor" AND "Mortality" OR "fatal" OR "case
fatality" OR "case fatality rate" AND "Neonate" OR "childhood"
OR "neonatal" OR "newborn" AND "Pneumonia" OR "hospital acquired
pneumonia") OR "community-acquired pneumonia" OR "ventilator
associated pneumonia" OR "early onset pneumonia" OR "late onset
pneumonia." Additionally grey literature search and snowballing
were also conducted to find out potentially relevant studies.
The authors were contacted in an attempt to retrieve missing
information on important methodological aspects or outcomes
measures.
Data extraction and quality assessment:
Considering inclusion and exclusion criteria, three review
authors (SM, MG, and TL) worked in two teams to screen, extract
data and quality assessment of identified literature. The
consensus for any discrepancies were sought through discussion
with senior reviewers (NSN, LL, and BTV). A Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA) chart
was generated to summarize the study selection process.
Characteristics were summarized and results were reported using
tables and accompanied by a descriptive summary that compared
and evaluated the methods and results of included studies. The
results of the search were managed and screened using Endnote
(v. x7). Microsoft Excel 2007 was utilized for data extraction.
Statistical analysis: Depending on
methodological hetero-geneity, a random-effects model was used.
The summary measures were pooled based on study design. A Forest
plot was generated and pooled estimates were reported with 95%
CIs. Based on the availability of data, a subgroup analysis was
also planned a priori with respect to study design, type of
neonatal pneumonia, study setting, and onset of pneumonia.
However, the subgroup analysis was not possible due to
non-availability of relevant data. For meta-analysis, data were
available only from two studies on VAP (ventilator-associated
pneu-monia) and meta-analysis was possible only for two factors
i.e., very low birth weight and prematurity. Depending on data
availability, a sensitivity analysis and meta-regression was
planned but could not be performed due to limited data.
Reporting bias could also not be assessed as included studies
were less than 10.
The reporting has been done in accordance
with the Preferred Reporting Items for Systematic reviews and
Meta-Analysis (PRISMA) guidelines [12] and the Meta-analysis of
Observational Studies in Epidemiology (MOOSE) guidelines [13].
Quality assessment was done at the study level using the
modified Quality Assessment Tool for Systematic Reviews of
Observational Studies (QATSO) tool [14]. STATA (v.13) was used
to perform statistical analyses.
RESULTS
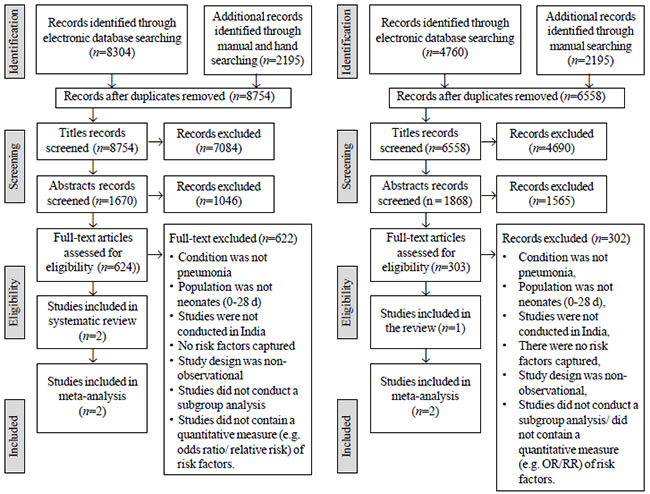
A total of 8754 citations were subjected to
title screening, and finally two articles were found to be
eligible and were included for the meta-analysis (Fig. 1).
For mortality predictors of neonatal pneumonia, a total of 6,955
citations were identified, of which, 303 articles were screened
for full text and only one article was eligible for inclusion (Fig.
2). Meta-analysis was not possible as there was only one
eligible study.
 |
|
Fig. 1 PRISMA flow chart
depicting the study selection process for risk factors
of neonatal pneumonia.
|
Fig. 2 PRISMA flowchart
depicting the study selection process for factors
associated with mortality.
|
For risk factors, two studies [15,16] were
included with data from a total of 194 neonates. For mortality
predictors, only one study [17] with 150 neonates was
included. Table I lists the characteristics of included
studies [18].
Both studies used (NNIS) 1996 guidelines in
conjunction with pediatric modification of the original center
for disease control guidelines. [15,16]. Both studies (risk
factors) found Klebsiella species as the most predominantly
isolated organism from the endotracheal aspirate of neonates
with ventilator associated pneumonia. None of the studies
reported the socio-demographic characteristics of neonates with
or without ventilator associated pneumonia.
Web Table I
describes in detail diagnostic criteria used for ventilator
associated pneumonia [15,16] and neonatal pneumonia [17]. The
study on mortality predictors did not specify the guideline
followed for diagnosis of neonatal pneumonia, the authors
reported the use of the National Neonatology Forum (NNF) to
diagnose ‘respiratory problems’. In both the studies [15,16] no
primary criteria for mechanical ventilation was provided in the
methodology; however, in one of the study results indicated four
conditions for mechanical ventilation namely pneumonia, apnea,
poor respiratory effort and Hyaline Membrane Disease [15].
In total, 11 risk factors were identified
from two studies and six of them were common across both
studies. Table I provides a risk factor profile of the
included studies [15, 16]. A random effects model was used for
the meta-analysis. Meta-analysis was carried out for only two
factors from two studies namely very low birthweight (VLBW) and
prematurity. In both the included studies, a significant
association was found between development of ventilator
associated pneumonia and duration of mechanical ventilation and
number of re-intubations; however, there was missing data and
attempts at reaching the authors were unsuccessful, therefore a
meta-analysis could not be performed for these factors.
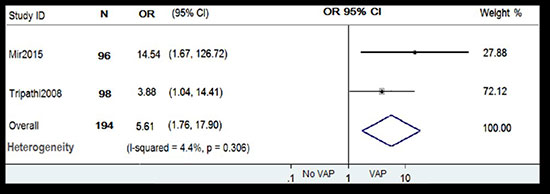
Pooled OR for very low birth weight from
random effects meta-analysis of two studies [15,16] is depicted
in Fig. 3. The forest plot show that neonates with VLBW
(<1500 g) were more likely to develop ventilator associated
pneumonia compared to neonates who were normal to low
birthweight (OR 5.61; 95% CI 1.76, 17.90). Very low birth weight
was found to be significant risk factor for development of
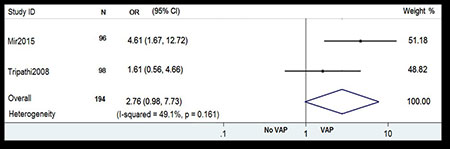
ventilator associated pneumonia. Pooled OR for prematurity is
depicted in Fig. 4 [15, 16]. The Forest plot shows
that neonates with estimated gestational age <37 week or
premature neonates were more likely to develop ventilator
associated pneumonia compared to term neonates (OR 2.76; 95% CI
0.98, 7.73).
 |
|
Fig. 3 Forest plot showing the
effect of very low birthweight on ventilator-associated
pneumonia.
|
 |
|
Fig. 4 Forest plot showing the
effect of prematurity on ventilator-associated
pneumonia.
|
Only one study [17] reported mortality
predictors to neonatal pneumonia. The authors did not specify
the list of independent and confounding variables considered as
predictors for fatality due to pneumonia in neonates. However,
they provided only P values for significant predictors
which they considered for multiple logistic regression. These
predictors included: <birthweight 2000 g, gestation <34 week,
age at presentation, lethargy, absent neonatal reflexes, shock,
Silverman score (4 to 6), FiO2>40%, pH<7.2, base excess >-10,
positive blood culture, C-reactive protein (CRP) positive, mean
alveolar arterial oxygen gradient (AaDO2) >250 mm Hg, mean
arterial alveolar tension ratio (a/A ratio) <0.25 and positive
ventilatory support. The authors found only AaDO2 gradient >250
mmHg as a significant predictor of mortality due to pneumonia
with respiratory distress in neonates (OR 71.1; 95% CI 1.1,
4395).
Publication bias could not be assessed as
there were fewer than 10 studies.
Web Table II depicts
quality assessment of studies using the QATSO Tool [14]. Four of
the five items on the scale were used to assess (i)
External validity, (ii) Reporting, (iii) Bias and
(iv) Confounding. However, no scoring was done. For
studies on risk factors, the measurement of pneumonia was only
found to be objective in one study [15] i.e., clinical records
or laboratory tests. Neither study reported any response rate.
Regarding the control of confounding factors when analyzing
associations, only one study partially accounted for this [15],
while the other did not report adequately on the handling of
variables during the analysis [16].
For study on mortality predictors [17] dose
response relationship could not be determined. The odds ratio in
the study was very large with large confidence intervals (71.1;
95% CI 1.1, 4395). No event rates were reported for both the
groups. Discrepancies exist in the numbers of participants
included in the study. The authors mention the presence of two
groups: respiratory distress with pneumonia and respiratory
distress without pneumonia. However, information on
socio-demographic characteristics, clinical and other important
exposure and confounding information for the two groups was
missing.
DISCUSSION
We conducted a series of systematic reviews
to determine the risk factors associated with development of
neonatal pneumonia and its mortality predictors in India.
Literature is widely available on pneumonia in general and on
neonatal sepsis. However there was near absence of data on
neonatal pneumonia particularly with respect to its risk factors
and mortality predictors in India. Only two studies were
included for risk factors and only one study on mortality
predictors of neonatal pneumonia. Meta-analysis for prematurity
and low birth weight was carried out and low birth weight was
found to be significant for the occurrence of neonatal
pneumonia. Only alveolar-arterial oxygen gradient (AaDO2) >250
mm Hg was found as a significant predictor of mortality due to
pneumonia with respiratory distress in neonates in present
review.
To the best of our knowledge this systematic
review is the first in India studying factors associated with
pneumonia and its mortality in neonates. A rigorous effort was
made to search the relevant studies without time restriction in
Indian context by means of conducting search on nine electronic
databases, hand searching, grey literature, by contacting
authors and in consultation with clinical experts to include
every possible study. Screening and data extraction was carried
out independently by two authors and discrepancies were resolved
by mutual discussion and by getting experts opinion.
Considering the limited evidence in this
review, studies on neonatal sepsis were included up to full text
screening to verify the presence or absence of a subgroup for
pneumonia but no clear underlying etiology as risk factor for
neonatal pneumonia was mentioned in these studies. Consequently
we have excluded studies where pneumonia was part of the
condition but further description for neonatal pneumonia was not
given separately.
Another limitation of the review was the lack
of response from authors of studies of neonatal sepsis that did
not explicitly provide data on the pneumonia component of their
sepsis cases. Due to lack of data in the papers, meta-analysis
for all the identified risk factors was not possible. Results
from our study on risk factors pertain only to cases of
ventilator associated pneumonia, which is a subgroup of neonatal
pneumonia, and therefore, the findings could not be extrapolated
to all the cases of neonatal pneumonia.
The major limitation for mortality predictors
of neonatal pneumonia was that we found limited evidence from a
single study that was not sufficient to conclude despite the
comprehensiveness of our search. One of the potential limitation
could be the language as we have restricted the search only to
articles published in English. Nonetheless, we might have not
missed any relevant studies on neonatal pneumonia as scientific
literature in India is mostly published in English.
Both the studies [15,16] in our review
investigated ventilator-associated pneumonia in neonates that
required mechanical ventilation for 48 hour or more as observed
in other studies [19-23]. In our review the incidence of
ventilator associated pneumonia ranged from 22 to 68 cases per
1000 MV days where as in another study from China it was 27.33
per 1,000 ventilator-days [21]. In contrast, in a study from
USA, VAP rates were as low as 6.5 and 4 per 1000 ventilator days
for patients with EGA <28 week and EGA >28 week, respectively
[22]. Both the included studies [15,16] found Klebsiella
species as the most predominantly isolated organism from the
endotracheal aspirate of neonates with VAP. Similarly in studies
from Egypt [23] and Western India [24], K. pneumoniae was
also found to be the most common organism.
High AaDO2 was found as a significant
predictor of mortality due to pneumonia. Similarly, in a study
from Bangladesh, high AaDO2 was one of the factors significantly
associated with change in antibiotics due to the worsening
condition of the neonates diagnosed with pneumonia [25].
However, they did not specify the limit to describe AaDO2 as
high whereas AaDO2 > 250 mm Hg was considered as high in the
included study [17]. In a multivariate logistic regression, VAP
was the single most important factor found to be significantly
associated with mortality, whereas marginally significant
association was found with presence of an arterial catheter
[22].
In contrast to our meta-analysis findings and
few other studies [19,20,22,26-28], there is one study [29]
where the occurrence of VAP was not associated with low
birth-weight (<1500 g). Results from meta-analysis of two
studies [15,16] found birth-weight of <1500 g as a significant
risk factor to develop VAP (OR 5.61; 95% CI 1.76, 17.90) Our
meta-analysis findings are comparable to a study from China
where low birth weight and premature infants had more chances of
developing VAP [28].
Differences in birth weight were observed
amongst different studies when it comes to defining weight at
birth as low. One study in our review [15] defined VLBW as less
than 1500 g. The other study [16] defined it as in between 1000
to 1500 g and excluded extremely low birth weight babies less
than 1000g weight. However, a study from Thailand reported a
neonatal birth weight less than 750g as an independent risk
factor for VAP [19] and another study established that VAP rates
were high among extremely preterm neonates but birth weight was
specified as £2000g
[22]. A retrospective observational study conducted in Taiwan
found that higher gestational age and weight at birth were
significantly associated in bringing down the VAP occurrence
[20].
Like other studies [27-29], duration of NICU
stay and MV were found as the risk factors but due to lack of
data, meta-analysis for these factors was not possible for our
review. Some intervention studies focused on the association of
the infection control program and VAP prevention [21, 23, 30]
and NICU stay [23]. However, one possible explanation for this
association could be the usage of humidifiers and closed-circuit
ventilation in NICU which provide a major source for growth of
microorganisms [28, 31]. Hence, NICU environment itself can be a
determining risk factor for development of VAP.
Risk factors that other studies have
attributed to neonatal pneumonia of early onset but were absent
from our review were antacid therapy [29], abnormal gastric
aspirate, and low APGAR score among high-risk infants [32].
However, it has also been reported that often risk factors are
absent in pneumonia of early onset, and that sudden onset of
preterm labor by its very nature; is considered as an important
risk factor [33].
Pneumonia is one of the leading causes of
death among neonates in India. Thus, factors that affect
neonatal mortality due to pneumonia and its occurrence are of
great importance for any effort to improve child survival.
However our review concludes that data and primary studies
itself is negligible to substantiate a holistic view on factors
associated with incidences and mortality of neonatal pneumonia.
There is no conclusive evidence on risk factors of neonatal
pneumonia other than ventilator associated pneumonia and hence
it is recognized with this review that neonatal pneumonia, which
comprises the majority of the burden of neonatal sepsis,
continues to be an understudied issue in the Indian neonatal
health scenario. To conclude, we can say that there is an
emergent need to prioritize research toward generating
evidence on neonatal pneumonia and determining factors for its
development and mortality.
Acknowledgments: The authors would like
to extend the gratitude to following persons for their guidance
and support throughout the development process of this
manuscript: Dr Manoj Das, Director Projects, The INCLEN Trust
International, New Delhi; Dr Anju Sinha, Deputy Director
General, Scientist ‘E’, Division of Child Health, Indian Council
of Medical Research, New Delhi; Dr KK Diwakar, Professor and
Head, Department of Neonatology, Associate Dean, Malankara
Orthodox Syrian Church Medical College, Kerala; Mrs Ratheebhai
V, Senior Librarian and Information Scientist, at Manipal School
of Communication, Manipal Academy of Higher Education (MAHE),
Manipal; Dr Ravinder M Pandey, Professor and Head, Department of
Biostatistics, All India Institute of Medical Sciences, New
Delhi; Dr B Shantharam Baliga, Professor, Department of
Paediatrics, Kasturba Medical College, Mangalore, Karnataka; Dr
Shrinivas Darak, Senior researcher, PRAYAS, Pune, Maharashtra;
Dr Unnikrishnan B, Associate Dean and Professor, Department of
Community Medicine, Kasturba Medical College, Mangalore. The
authors would like to thank Dr. Ravishankar N, Assistant
Professor, Department of Data Science, Prasanna School of Public
Health (PSPH), MAHE, Manipal for conducting meta-analysis.
Ethics clearance: Institutional
Ethics Committee, Manipal Academy of Higher Education, Manipal;
February 16, 2015.
Contributors: NSN: principal
investigator for the project and guarantor for this article. He
conceptualized the research idea and provided overall technical
guidance; LESL: co-investigator for the project. conceptualized
the research idea and provided overall technical guidance. In
addition, LL helped in developing search terms; VSD: drafted the
manuscript and contributed in drafting the full study report to
the funder; SM: conducted the search, piloted the study
selection process, drafted and piloted the data extraction form,
selected studies, extracted data, performed risk of bias,
synthesized data, and drafted the full study report to the
funder; MAG: conducted the search, piloted the study selection
process, drafted and piloted the data extraction form, selected
studies, extracted data, performed risk of bias, synthesized
data, and drafted the full study report to the funder; TL:
conducted the search, piloted the study selection process,
selected studies, conducted hand searching, extracted data,
performed risk of bias, synthesized data, and drafted the full
study report to the funder; BTV: administrative
coordinator for the project, and conceptualized
the research idea. She has also provided technical guidance
throughout the project, during protocol development, finalizing
the full report and addressing the project expert comments. All
authors approved the final version of manuscript, and are
accountable for all aspects related to the study.
Funding: This work was supported
by Bill and Melinda Gates Foundation through The INCLEN Trust
International (Grant number: OPP1084307). The funding source had
no contribution in study design, implementation, collection and
interpretation of data and report writing. Competing
interests: None stated.
REFERENCES
1. Child Mortality Estimates: Global and
regional child deaths by cause. Accessed April 04, 2019.
Available from: http://data. unicef.org
2. Duke T. Neonatal pneumonia in
developing countries. Arch Dis Child Fetal Neonatal Ed.
2005; 90:F211–FF219.
3. Ghimire M, Bhattacharya S, Narain J.
Pneumonia in South-East Asia Region: Public health
perspective. Indian J Med Res. 2012; 135:459.
4. Choudhury AM, Nargis S, Mollah AH, et
al. Determination of risk factors of neonatal pneumonia.
Mymensingh Med J. 2010;19:323-9.
5. Yang L, Zhang Y, Yu X, Luo M.
Prevalence and risk factors of neonatal pneumonia in China:
A longitudinal clinical study. Biomed Res. 2018; 29:57-60.
6. Almirall J, Serra-Prat M, Bolíbar I,
et al. Risk factors for community-acquired pneumonia in
adults: A systematic review of observational studies.
Respiration 2017; 94:299-311.
7. Liu B, Li SQ, Zhang SM, et al. Risk
factors of ventilator-associated pneumonia in pediatric
intensive care unit: A sys-tematic review and meta-analysis.
J Thorac Dis. 2013; 5:525-31.
8. Nair NS, Lewis LE, Godinho M, et al.
Risk factors for neonatal pneumonia in India: A systematic
review and meta-analysis. PROSPERO 2016 CRD42016044019.
Available from:https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD
42016044019
9. Nair NS, Lewis LE, Lakiang T, et al.
Risk factors for mortality due to neonatal pneumonia in
India: A systematic review and meta-analysis. PROSPERO 2016
CRD 42016045398. Available from:https://www.crd.york.ac.uk/prospero/display_record.php?
ID=CRD42016045398
10. Nair NS, Lewis LE, Godinho M, et al.
Factors associated with neonatal pneumonia in India:
Protocol for a systematic review and planned meta-analysis.
BMJ Open. 2018; 8: e018790.
11. Nair S, Lewis LE, Lakiang T, et al.
Factors associated with mortality due to neonatal pneumonia
in India: A protocol for systematic review and planned
meta-analysis. BMJ Open. 2018;8:e018790.
12. Liberati A, Altman DG, Tetzlaff J, et
al. The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: explanation and elaboration. Ann Intern Med.
2009;151:W65-94.
13. Stroup DF, Berlin JA, Morton SC, et
al. Meta-analysis of observational studies in epidemiology:
A proposal for reporting. JAMA. 2000; 283:2008-12.
14. Wong WC, Cheung CS, Hart GJ.
Development of a quality assessment tool for systematic
reviews of observational studies (QATSO) of HIV prevalence
in men having sex with men and associated risk behaviours.
Emerg Themes Epidemiol. 2008; 5:23.
15. Tripathi S, Malik GK, Jain A, et al.
Study of ventilator associated pneumonia in neonatal
intensive care unit: Characteristics, risk factors and
outcome. Internet J Med Update. 2010; 5:12-19.
16. Mir ZH, Ali I, Qureshi OA, et al.
Risk factors, pathogen profile and outcome of ventilator
associated pneumonia in a neonatal intensive care unit. Int
J Contemp Pediatr. 2015; 2:17-20.
17. Mathur NB, Garg K, Kumar S.
Respiratory distress in neonates with special reference to
pneumonia. Indian Pediatr. 2002; 39:529-37.
18. Schünemann HJ, Oxman AD, Higgins JP,
et al. Presenting results and ‘Summary of findings’ tables.
In: Higgins J, Green S, editors. Cochrane Handbook
for Systematic Reviews of Interventions. Version 5.1.0. The
Cochrane Collaboration, 2011. Available from:
www.cochranehandbook.org
19. Thatrimontrichai A, Rujeerapaiboon N,
Janjindamai W, et al. Outcomes and risk factors of
ventilator-associated pneumonia in neonates. World J Pediatr.
2017; 13:328-34.
20. Lee PL, Lee WT, Chen HL.
Ventilator-Associated Pneu-monia in Low Birth Weight
Neonates at a Neonatal Intensive Care Unit: A Retrospective
Observational Study. Pediatr Neonatol. 2017; 58:16-21.
21. Zhou Q, Lee SK, Jiang SY, et al.
Efficacy of an infection control program in reducing
ventilator-associated pneumonia in a Chinese neonatal
intensive care unit. Am J Infect Control. 2013; 41:1059-64.
22. Apisarnthanarak A, Holzmann-Pazgal G,
Hamvas A, et al. Ventilator-associated pneumonia in
extremely preterm neonates in a neonatal intensive care
unit: Characteristics, risk factors, and outcomes.
Pediatrics. 2003; 112:1283-9.
23. Azab SF, Sherbiny HS, Saleh SH, et
al. Reducing ventilator-associated pneumonia in neonatal
intensive care unit using "VAP prevention Bundle": A cohort
study. BMC Infect Dis. 2015; 15:314.
24. Amin AJ, Malam PP, Asari PD, et al.
Sensitivity and resistance pattern of antimicrobial agents
used in cases of neonatal sepsis at a tertiary care center
in western India. Int J Pharm Sci Res. 2016; 7:3060-67.
25. Islam MM, Hossain MM, Al Mamun MA, et
al. Risk factors which affects the change of antibiotics in
neonatal pneumonia observed in a tertiary care hospital.
Northern Int Med Coll. 2014; 6:21-4.
26. Medeiros FD, Alves VH, Valete CO, et
al. Invasive care procedures and neonatal sepsis in newborns
with very low birth weights: A retrospective descriptive
study. Online Braz. J Nurs. 2016; 15:704-12.
27. Afify M, AI-Zahrani S, Nouh MA. Risk
Factors for the Development of Ventilator – Associated
Pneumonia in Critically-ill Neonates. Life Sci J. 2012;
9:302-7.
28. Tan B, Zhang F, Zhang X, et al. Risk
factors for ventilator-associated pneumonia in the neonatal
intensive care unit: a meta-analysis of observational
studies. Eur J Pediatr. 2014; 173:427-34.
29. Afjeh SA, Sabzehei MK, Karimi A, et
al. Surveillance of ventilator-associated pneumonia in a
neonatal intensive care unit: Characteristics, risk factors,
and outcome. Arch Iran Med. 2012; 15:567-71.
30. Ryan RM, Wilding GE, Wynn RJ, et al.
Effect of enhanced ultraviolet germicidal irradiation in the
heating ventilation and air conditioning system on
ventilator-associated pneu-monia in a neonatal intensive
care unit. J Perinatol. 2011; 31:607-14.
31. Garland JS. Strategies to prevent
ventilator-associated pneumonia in neonates. Clin Perinatol.
2010; 37:629-43.
32. Mahendra I, Retayasa IW, Kardana IM.
Risk of early onset pneumonia in neonates with abnormal
gastric aspirate. Pedia-trica Indonesiana. 2008;48:110-13.
33. Webber S, Wikinson AR, Lindsell D, et al. Neonatal
pneu-monia. Arch Dis Child. 1990; 65:207-11.
|
|
|
 |
|

