|
|
|
Indian Pediatr 2021;58:1040-1045 |
 |
Predictors of Mortality
in Neonatal Pneumonia: An INCLEN Childhood Pneumonia Study
|
|
C Suresh Kumar, 1 Sreeram
Subramanian,2 Srinivas
Murki,3 JV Rao,4
Meera Bai,5 Sarguna
Penagaram,5 Himabindu Singh,6
Nirupama Padmaja Bondili,7
Alimelu Madireddy,6 Swapna
Lingaldinna,6 Srikanth Bhat,4
Bharadwaj Namala6
From Departments of Pediatrics, 1Institute of Child Health, Niloufer
Hospital, Osmania Medical College, and 4Gandhi Hospital and Medical
College; Departments of Neonatology, 2Paramitha and NeoBBC Children
Hospital, 3Fernandez Hospital, and 6Niloufer Hospital and Osmania
Medical College; and Departments of Microbiology, 5Osmania Medical
College and SRRIT&CD, and 7Fernandez Hospital, Hyderabad, Telangana.
Correspondence to: Dr Sreeram Subramanian, Neonatologist, Paramitha
and NeoBBC Children Hospital,
Hyderabad, Telangana.
Email: [email protected]
Received: June 08, 2020;
Initial review: August 25, 2020;
Accepted: February 10, 2021.
|
Background:
Neonatal pneumonia contributes significantly to mortality due to
pneumonia in the under-five age group, but the predictors of mortality
are largely unknown.
Objective: To evaluate the clinical and
microbiological charac-teristics and other risk factors that predict
mortality in neonates admitted with pneumonia in tertiary care centres.
Study design: Prospective observational cohort
study.
Participants: Term and preterm (32 weeks to 36
6/7 weeks) neonates (<28 days of life) admitted with clinical and
radiological features suggestive of pneumonia.
Intervention: Baseline sociodemographic data,
clinical details, blood culture and nasopharyngeal swabs for virologic
assay (RT PCR for RSV, Influenza) were collected at admission and the
neonates were observed throughout their hospital stay.
Outcome: The primary outcome was predictors of
mortality in neonatal pneumonia.
Results: Five hundred neonates were enrolled in
the study. Out of 476 neonates with known outcomes, 39 (8.2%) died. On
multivariate analysis, blood culture positive sepsis was independently
associated with mortality (adjusted OR 2.51, 95% CI1.23 to 5.11; P-0.01).
Conclusion: Neonates with blood culture positive
pneumonia positive are at a higher risk of death.
Key words: Burden, Early onset sepsis, Outcome, Risk Factors.
|
|
W
ith a mortality rate
of 37/1000 live births,
India ranks among the top five nations
with high under five mortality rates.
Neonatal mortality, comprising 60% of under-five mortality, is
markedly higher than that in any high-income group country [1].
Neonatal sepsis, the third most common cause of neonatal deaths,
has a significant impact on long term neurodevelopment. Neonatal
pneumonia alone accounts for 2% (0.136 million) of under-five
mortality in children in the world [2].
Though the incidence of neonatal sepsis among
NICU admissions in our country is reported to be 14.3%, the
occurrence of neonatal pneumonia and the factors predicting
mortality are not well studied [3-7]. Ventilator associated
pneumonia is common among preterm, low birth weight or
mechanically ventilated newborns [7]. In this study, we evaluate
the clinical and microbiological characteristics and other risk
factors that predict mortality in term and preterm (32 to 36
6/7 weeks) neonates
admitted with pneumonia, in tertiary care public sector
pediatric hospitals, catering predominantly to outborn neonates.
METHODS
This multi-centre, prospective, cohort study,
conducted in two tertiary level public sector hospitals,
included neonates having tachypnea, respiratory distress (chest
retractions/grunting) and evidence of pneumonia on chest X-ray
[8]. Neonates having meconium aspiration syndrome, or
respiratory distress developing within first 2 hours of life and
improving within 12 hours of life or those with major congenital
malformations or those admitted for >24 hours in another
hospital or received antibiotics prior to admission, were
excluded. Nodular or coarse, patchy non-homogenous infiltrates,
air broncho-gram, lobar, multi lobar or segmental consolidation
were considered as radiological evidence of pneumonia. Eligible
infants were enrolled after obtaining consent from either
parent. Blood culture and nasopharyngeal aspirates was taken at
admission and case details, clinical course and the outcome data
were recorded in a predesigned proforma. The clinical staff were
trained to interpret X-rays and the diagnosis was made by
the resident involved in study, was confirmed by a consultant,
and the neonates were managed as per standard treatment
guidelines [9]. Echocardiography was done only when clinically
indicated.
The primary outcome was predictors of
mortality in neonatal pneumonia. Predictors evaluated included
socio-demographic factors, maternal age, maternal fever, parity,
mode of delivery, the clinical features at admission [10] and
during the course of hospitalization as well as microbiological
characteristics of the isolates. The secondary outcomes included
overall blood culture positivity rate in neonatal pneumonia, the
distribution of microbiological causes, the need for higher
respiratory support and complications of pneumonia.
To ensure quality, the microbiological
samples were processed at a NABL accredited laboratory with an
active external quality assessment program. Apart from this, the
unusual bacterial organisms and fungal isolates were confirmed
using MALDI TOF assay at an another NABL accredited laboratory.
Further, interlab comparison of 10% of all positive and negative
viral isolates were done. All data collected were cross-verified
by the site investigators periodically.
Assuming a 10% prevalence of any of the
predictors, an odds ratio of 2.5 for mortality and a mortality
rate of 8% in neonatal pneumonia [11], the number of babies
expected to die due to pneumonia was 121. To realize this
target, 1500 neonates needed to be enrolled. In view of the slow
recruitment and time constraints, an interim analysis was done
on the data until June 2019 (353 neonates were enrolled till
then) and using the proposed predictors, the sample size was
revised to 606. Nasopharyngeal swabs were also collected form
100 healthy term neonates to look at the pattern of asymptomatic
viral colonisation.
The study was approved by the individual
ethics committees of the participating hospitals.
Statistical analysis: Comparison of
categorical variables was done by Chi square test, while
continuous variables were compared using Student t-test.
Risk ratio along with 95% CI was presented. Univariate and
multivariate binary logistic regression analysis was performed
to test the association between possible risk factors and
outcome variables. Variables with statistical significance (P
value <0.1) in univariate analysis were used to compute
multivariate regression analysis. Adjusted odds ratio with 95%
CI was calculated, taking P value < 0.05 as statistically
significant. All statistical analysis was done on IBM SPSS
version 22.
RESULTS
Out of a total of 915 eligible neonates, 500
were enrolled (Fig. 1). The mean (SD) birthweight of the
neonates was 2635.16 (533) g with 8 (1.6%) being very low
birthweight (VLBW). The mean (SD) gestational age was 37.29
(1.9) weeks, with 130 (25%) being preterm. Most of the families
(52%) belonged to upper lower socioeconomic class followed by
lower middle socioeconomic class (41.7%). Out of 476 neonates
with known outcomes, 39 (8.2%) died. The comparison of
parameters between surviving and non-surviving neonates is shown
in Table I. There were significantly higher proportions
of VLBW and preterm neonates in the non-surviving group,
compared to survivors.
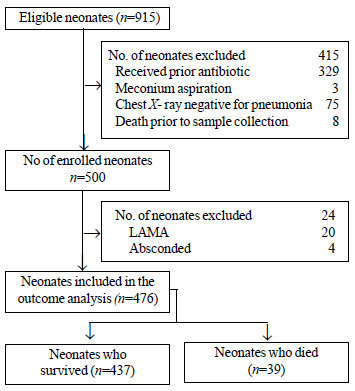 |
|
Fig. 1 Study flow chart.
|
Table I Comparison of Sociodemographic, Antenatal and Birth Parameters Between Surviving and Non-Surviving Neonates
| Parameters |
Non-survivors (n=39) |
Survivors (n=437) |
Relative risk (95%CI) |
|
Birthweight (g)a,e
|
2403 (622) |
2639 (515) |
235.6 (63.4 , 407.8 )c |
|
Birthweight ³1500 ge
|
3 (7.7) |
5 (1.1) |
6.29 (1.88, 21.07) |
| Gestational age (wk)a |
36.72 ( 2.69) |
37.29 (1.93) |
0.88 (0.77, 1.01) |
| Preterm birth |
16 (41.0) |
111 (25.4) |
1.91 (1.04, 3.50) |
| Male gender |
32 (82.0) |
331 (75.7) |
1.42 (0.65, 3.14) |
| Vaginal delivery |
21 (53.8) |
218 (49.9) |
1.14 (0.61, 2.14) |
| Primigravida mother |
38 (97.4) |
382 (87.4) |
4.99 (0.69, 36.32) |
|
Antenatal visitsb |
4 (3,4) |
3 (3,4) |
0.95 (0.76, 1.19) |
|
Maternal feverd |
1 (2.6) |
11 (2.5) |
1.07 (0.15, 7.79) |
| Apgar score (1 min)b |
5 (5,5) |
5 (5,5) |
0.81 (0.54, 1.19) |
| Apgar score (5 min)f |
8 (8,8) |
8 (8,8) |
0.65 (0.47, 0.89) |
|
Weight at admission (g)a,e
|
2382.44 (628.47) |
2691.68 ( 569.66) |
309.12 (120.6, 497.6)c |
|
Age at admission (h)a,e |
136.46 ( 173.07) |
301.67 (232.74) |
165.2 (90.03, 240.3)c |
|
Hospital stay a,g |
5.9 (6.8) |
7.81 ( 5.6) |
1.9 (-0.02, 3.84) |
|
Values in n (%),amean (SD) or bmedian (IQR). cmean
difference (95% CI). dmaternal fever within 1 week prior
to delivery. eP<0.001, fP=0.007, gP=0.05. |
Onset of symptoms occurred at a mean of 5.6
days of life in the neonates who died, compared to 12.5 days in
those who survived [mean difference 6.9 (95% CI 3.7, 10); P<0.001].
The most common presenting symptom was difficulty in feeding
seen in 219 (46%)] neonates, followed by fever, noted
in 110 (23%) of the neonates. The most common sign was
tachypnea, mean (SD) respiratory rate being 63.7 (6.8) breaths
per minute and the median Silverman Anderson score at admission
was 4 (IQR 3,6). At admission, 302 (60%) neonates required
oxygen, with 143 (28%) being started on CPAP, and 55 (11%)
requiring intubation. The comparison of clinical features and
course between surviving and non-surviving neonates is shown in
Table II
Table II Comparison of Clinical Features and Course of Illness Between Surviving and
Non-Surviving Neonates and Survivors
| Parameters |
Non-survivors (n=39) |
Survivors (n=437) |
Relative risk (95%CI) |
| Cough |
2 (5.13) |
65 (14.87) |
0.34 (0.08, 1.40) |
| Running nose (cold) |
1 (2.56) |
38 (8.7) |
0.29 (0.04, 2.15) |
| Fever |
7 (17.95) |
103 (23.57) |
0.73 (0.32, 1.66) |
| Breathing difficulty |
33 (84.62) |
398 (91.08) |
0.58 (0.25, 1.39) |
| Apneab |
5 (12.82) |
14 (3.2) |
3.35 (1.31, 8.57) |
| Cold to
touchc |
7 (17.95) |
26 (5.95) |
2.99 (1.32, 6.79) |
| Vomiting |
4 (10.26) |
32 (7.32) |
1.43 (0.51, 4.02) |
| Diarrhea |
0 |
6 (1.37) |
- |
| Feeding difficulty |
22 (56.41) |
197 (45.08) |
1.53 (0.81, 2.88) |
| Seizuresb |
8 (20.5) |
33 (7.6) |
2.74(1.26, 5.97) |
| Movement
only with stimulationb |
10 (25.64) |
46 (10.53) |
2.58 (1.26, 5.29) |
| Heart rate >180/min |
4 (10.26) |
46 (10.53) |
0.96 (0.34, 2.71) |
| SAS
scorea,b |
5 (4, 6) |
4 (3,5) |
1.278 (1.054, 1.550) |
| Grunting |
26 (66.67) |
226 (51.72) |
1.77 (0.91 to 3.45) |
| CFT>3 seconds |
5 (12.82) |
48 (10.98) |
1.19 (0.47, 3.04) |
| Temp >37.5 ºC |
4 (10.2) |
101 (23) |
0.40 (0.16, 1.03) |
| Temp <36.5 ºC |
4 (10.26) |
17 (3.89) |
2.48 (0.88, 6.99) |
| Cyanosisc |
8 (20.51) |
16 (3.66) |
4.71 (2.16, 10.24) |
| SpO2< 90% |
23 (59) |
205 (46.9) |
1.56 (0.84, 2.88) |
| Bulging
anterior fontanelle |
2 (5.13) |
23 (5.26) |
0.91 (0.22, 3.78) |
| Lethargy |
18 (46.15) |
150 (34.32) |
1.56 (0.83, 2.94) |
| Abdominal
distensionb |
6 (15.38) |
22 (5.03) |
2.95 (1.24, 7.05) |
| Hepatomegaly |
4 (10.26) |
51 (11.67) |
1.19 (0.47, 3.04) |
| More than one skin pustule |
1 (2.56) |
1 (0.23) |
6.55 (0.90, 47.73) |
| Respiratory support at admission |
|
|
|
| Oxygend |
14 (35.9) |
272 (62.24) |
0.37 (0.19, 0.69) |
| Intubationc |
16 (41.03) |
38 (8.7) |
6.28(3.06, 12.86) |
| CPAP |
9 (23.08) |
127 (29.06) |
1.36(0.6, 3.14) |
|
Values in (%) or amedian (IQR). bP=0.01; cP<0.001;
P=0.002. CPAP: continous positive airway pressure; SAS:
Silverman Anderson score. |
While blood culture positivity rate was
significantly higher among neonates who died, viral isolates in
the nasopharynx was significantly higher among survivors, RSV B
being the most common. (Table III). Overall blood culture
positivity rate was 19.2%, Gram negative organisms were isolated
in 45 (47%) and Gram-positive organisms in 23 (24%) neonates.
Klebsiella was the commonest organism isolated and was seen in
22 neonates (23%). While 27 (28%) neonates showed fungal growth
with Candida species,190 (38%) neonates were positive for
viral PCR. Among 100 healthy term neonates, 7 were found to have
asymptomatic viral colonisation (Influenza B – 5, H1N1 – 1, both
influenza A and B -1)
Table III Comparison of Microbiological Parameters Between Surviving and Non-Surviving Neonates
| Parameters |
Non-survivors (n=39) |
Survivors (n=437) |
Relative risk (95%CI) |
P value |
| Blood culture positive |
15 (38.4) |
78 (17.8) |
2.63 (1.38 - 5.01) |
0.003 |
| Gram positive |
2 (5.1) |
20 (4.5) |
0.52 (0.12 - 2.25) |
0.38 |
| Gram negative |
8(20.5) |
38 (8.7) |
0.49 (0.06 - 3.71) |
0.49 |
| Fungal |
5(12.8) |
20 (4.5) |
1.46 (0.41 - 5.12) |
0.56 |
| Viral PCR positive |
4 (10.3) |
177 (40.5) |
0.18 (0.06 - 0.525) |
0.001 |
| RSV B |
3 (7.7) |
118 (27) |
0.24 (0.07 - 0.79) |
0.01 |
|
Values in n (%). RS: respiratory syncytial virus. |
On multivariate analysis, positive blood
culture (adjusted OR 2.51, 95% CI 1.23 to 5.11; P=0.01)
emerged as the independent predictor of mortality in neonates
with pneumonia.
DISCUSSION
In this study the mortality rate due to
neonatal pneumonia was found to be 8.2%. The blood culture
positivity was an independent predictor of mortality, though the
type of organism did not affect mortality. The mortality rate is
less than that reported (12%) in the multicenter national
neonatal perinatal database report [11]. In the DeNIS cohort [3]
though the overall blood culture positivity among neonates with
pneumonia was almost similar to our study (15% vs 19%,
respectively), the mortality rate was lower (45% vs 16%,
respectively) and was probably due to differences in inclusion
criteria and the higher prevalence of multidrug resistant
organisms. In the present study, 50% of the bacterial isolates
were Gram negative, Klebsiella being the commonest
organism reflecting community prevalence. On the other hand,
two-third of the culture positive isolates were Gram negative in
DeNIS study, Acinetobacter being the commonest isolate
[3]. Streptococcus pneumoniae, increasingly found in
possible serious bacterial infections (pSBI) among young
infants, has been reported to contribute to mortality [12].
However, we did not isolate any S. pnemoniae, possibly
due to inherent difficulty in isolating in blood cultures and
the need for additional techniques. The overall microbiological
yield was 53%, which is double than that reported in
community-acquired serious bacterial infections [12].
The incidence of community-acquired fungal
pneumonia in our cohort, confirmed by molecular diagnosis (MALDI
TOF), was very high (25% of culture positive), compared to other
studies [3,12], although this did not translate into higher
mortality. This is intriguing as none of the neonates received
any antibiotic nor were admitted in any hospital prior to
enrolment.
The viral positivity rate in our study (38%)
was similar to that of a community-based surveillance study
(42%) involving infants with respiratory illness from
Bangladesh, but neonatal pneumonia constituted only 11% in their
cohort [13]. Several hospital based studies in neonates, from
Asia, have reported 30% incidence of viral lower respiratory
tract infections, especially due to RSV [14]. The in-hospital
case fatality rate of viral pneumonia was 0.2% which is
significantly lower than the reported incidence in LMIC
countries [5·3% (95% CI 2·8 to 9·8)] possibly due to better
management in tertiary care centers [15]. Moreover, neonates
with viral pneumonia had higher body weight and presented at a
later age in the neonatal period, which could possibly explain
better outcome., The most common viral isolate in the present
study was RSV, consistent with the global burden, but unlike the
usual pattern, type B strain was dominant, which could be
another reason for better survival [14-16].
The symptoms and signs used in our study were
similar to those in integrated management of neonatal and
childhood illness (IMNCI) and young infant study [17,18]. Though
they have been shown to predict the occurrence of pBSI, our data
failed to show their association with mortality.
The strength of our study was the use of a
very strict case definition, which, to the best of our
knowledge, is the first and largest of its kind. The limitation
of the study was the inability to enroll the originally planned
1500 neo-nates, due to logistic constraints. Other etiological
agents like Mycoplasma, Chlamydia, and Pneumococcus requiring
special techniques for isolation, were not evaluated. The
investigators involved in the inter-pretation of X-rays
were not blinded to clinical features.
In conclusion we found blood culture
positivity in neonatal pneumonia as an independent predictor of
mortality. The role of fungus in community acquired neonatal
pneumonia needs further exploration and there is need to be
vigilant and consider early antifungal therapy especially in
those who do not seem to respond. The high incidence of viral
pneumonias in our study emphasizes the need to consider
nasopharyngeal swab in the neonatal pneumonia work up.
Vaccination against RSV immediately after birth may be a
potential strategy to lower the burden of neonatal pneumonia.
[19]
Acknowledgements: Technical advisory
group constituting Prof Dr Lalitha Krishnan, Prof Dr Siddharth
Ramji and Prof Dr Ramesh Agarwal for their critical appraisal of
the project and for providing technical guidance. INCLEN,
especially Dr Manoj K Das, for providing continuous technical
and logistic support for the study. Dr Murali Reddy and his team
from Beyond P value for providing statistical assistance. We
also thank the project coordinator Mrs. Pavani Soujanya for
supervising and coordinating the project, and also analyzing the
viral isolates.
Ethics clearance: (i)
Ethics committee of Osmania medical college, Hyderabad; Reg No
ECR/300/Inst/AP/2013 Date of approval June 23, 2015; (ii)
Ethics committee of Gandhi Medical college, Hyderabad,
ECR/180/Inst/AP/2013- October 13, 2015; and (iii) Ethics
committee of Fernandez hospital, Hyderabad; ECR/933/Inst/TG/2017
– December 09, 2015.
Contributors: SK, SS, SM, JVR, MB:
involved in the conception, design of the project; SK,SS,SM
were also involved in data analysis, drafting of manuscript; MB,
SP, NPB: designed and conducted the microbiological aspects of
the study; HS, AM, SL, SB, BN: involved in case enrolment and
supervision. All the authors were involved in critical appraisal
and have reviewed and approved the manuscript.
Funding: This work was supported by Bill
and Melinda Gates Foundation through The INCLEN Trust
International (Grant number: OPP1084307). The funding source had
no contribution in study design, implementation, collection and
interpretation of data and report writing. Competing
interests: None stated.
|
WHAT IS ALREADY KNOWN?
•
Predictors of moratality
in neonatal pneumonia are largely unknown.
WHAT THIS STUDY ADDS?
•
Blood culture positivity independently predicts
mortality in neonatal pneumonia.
•
High prevalence of RSV B and Candida was seen
in neonatal pneumonia.
|
REFERENCES
1. United Nations Inter-agency Group for
Child Mortality Estimation (2019). Accessed April10,2020.
Available from: https://childmortality.org/data/India
2.. Liu L, Oza S, Hogan D, et al. Global,
regional, and national causes of child mortality in 2000-13,
with projections to inform post-2015 priorities: An updated
systematic analysis. Lancet. 2015;385:430-40.
3. Investigators of the Delhi Neonatal
Infection Study (DeNIS) Collaboration Characterisation and
antimicrobial resistance of sepsis pathogens in neonates
born in tertiary care centres in Delhi, India: A cohort
study. Lancet Glob Health. 2016;4: e752-e60.
4. Duke T. Neonatal pneumonia in
developing countries. Arch Dis Child Fetal Neonatal Ed.
2005;90:F211-19.
5. Dutta S, Reddy R, Sheikh S, et al.
Intrapartum antibiotics and risk factors for early onset
sepsis. Archi Dis Child Fetal Neonatal Ed. 2010 Mar
1;95:F99-103.
6. Chacko B, Sohi I. Early onset neonatal
sepsis. Indian J Pediatr. 2005;72:23-26.
7. Hooven TA, Polin RA. Pneumonia. Sem
Fetal and Neonatal Med. 2017:22:206-13.
8. Mathur NB, Garg K, Kumar S.
Respiratory distress in neonates with special reference to
pneumonia. Indian Pediatr. 2002;39:529-37.
9. Workbook On CPAP. Science evidence and
practise. Deorari AK, Kumar P, Murki S editors. 4th edition.
Accessed April 01, 2020. Available from:
https://www.newbornwhocc.org/CPAP-book-2017.html
10. Goldstein B, Giroir B, Randolph A;
Inter-national Pediatric Sepsis Consensus Conference:
Definitions for Sepsis and Organ Dysfunction in Pediatrics.
Pediatr Crit Care Med. 2005;6:2-8.
11. Report of National Neonatal Perinatal
Database (NNPD) 2002-2003. Accessed January 20, 2014.
Available from: http://www.newbornwhocc.org/nnpo.html
12. Saha SK, Schrag SJ, El Arifeen S, et
al. Causes and incidence of community-acquired serious
infections among young children in south Asia (ANISA): an
observational cohort study. Lancet. 2018;392:145-59
13. Farzin A, Saha SK, Baqui AH, et al.
Population-based incidence and etiology of
community-acquired neonatal viral infections in Bangladesh.
Pediatric Infect Dis J. 2015;34: 706-11.
14. Zhang Y, Yuan L, Zhang Y, et al.
Burden of respiratory syncytial virus infections in China:
Systematic review and meta-analysis. J Glob Health.
2015;5:020417.
15. Shi T, McAllister DA, O’Brien KL, et
al. Global, regional and national disease burden estimates
of acute lower respiratory infections due to respiratory
syncytial virus in young children in 2015: A systematic
review and modelling study. Lancet. 2017;390:946-58.
16. Rodriguez-Fernandez R, Tapia LI, Yang
C-F, et al. Respiratory syncytial virus genotypes, host
immune pro-files, and disease severity in young children
hospitalized with bronchiolitis. J Infect Dis.
2018;217:24-34.
17. Ingle G K, Malhotra C. Integrated
management of neonatal and childhood illness: An overview.
Indian J Comm Med. 2007;32:108-10.
18. The Young Infants Clinical Signs
Study Group. Clinical signs that predict severe illness in
children under age 2 months: a multicentre study. Lancet.
2008;371:135-42.
19. Giersing BK, Modjarrad K, Kaslow DC, et al. Report from
the World Health Organization’s Product Development for Vaccines
Advisory Committee (PDVAC) meeting, Geneva, 7-9th Sep 2015.
Vaccine. 2016;34:2865-69.
|
|
|
 |
|

