|
|
|
Indian Pediatr 2019;56: 972 -974 |
 |
Life-threatening Hypercalcemia as the First Manifestation of
Acute Lymphoblastic Leukemia
|
|
Kakali Roy1,
Rajni Sharma1*,
Manisha Jana2
and Vandana Jain1
From Departments of 1Pediatrics
and 2Radiology, AIIMS, New Delhi, India.
Email:
[email protected]
|
|
Hypercalcemia of malignancy, usually
reported in adults in advanced stages, is rare in children. A 4-year-old
boy presented with intermittent episodes of severe hypercalcemia, which
improved with intravenous hydration therapy, furosemide and
bisphosphonates as the initial manifestation of occult acute
lymphoblastic leukemia. Pediatricians should rule out hematological
malignancy in patients with severe hypercalcemia.
Keywords: Constipation, Diabetes insipidus.
|
|
S
evere hypercalcemia (>14 mg/dL) is rare in
children but can result in coma, arrhythmias and death. In adults, the
most common cause of severe hypercalcemia is malignancy (advanced
stages), which portends a poor prognosis. Hypercalcemia of malignancy
has been rarely reported in children [1]. We present a boy with
life-threatening hypercalcemia who was diagnosed with occult acute
lymphoblastic leukemia (ALL).
A 4-year-old boy presented with recurrent vomiting,
polyuria, polydipsia, extreme irritability, irrelevant talk,
constipation and right leg immobility with the history of low back pain
for two months. The child was in severe dehydration with altered
sensorium, high blood pressure (>99 th
centile) without any neurological deficit, hepatosplenomegaly or
lymphadenopathy. On laboratory investigations, serum calcium was 19.2
mg/dL (ionized 8.2 mg/dL), phosphate 3.7 mg/dL, albumin 3.7 g/dL,
alkaline phosphatase 389 IU/L, high urinary calcium: creatinine ratio,
25(OH)D 40 nmol/L (normal 50-250), increased 1,25(OH)2D
271 pmol/L (normal 52-117); and normal venous blood gas, renal function
and serum magnesium. He was managed with hydration with double
maintenance fluid and intravenous furosemide. Pamidronate (1 mg/kg) was
administered over 4 hours by intravenous infusion. The serum calcium
improved to 9.2 mg/dL and subsequently fell to 6.8 mg/dL over the next 3
days requiring oral calcium supplementation. A diagnosis of primary
hyperparathyroidism was considered based on serum PTH level of 106 pg/ml
(15-68.3) and doubtful PTH gland adenoma on ultrasound of neck.
Sestamibi scan, 4D CT neck and 4D MRI neck did not identify any
parathyroid gland abnormality. A repeat serum PTH was reported low at
1.3 pg/mL with normal calcitonin (<2 pg/mL, normal 0-18.2). Work up for
PTH-independent causes of hypercalcemia including granulomatous disease
(tuberculosis and angiotensin-converting enzyme levels for sarcoidosis)
was normal.
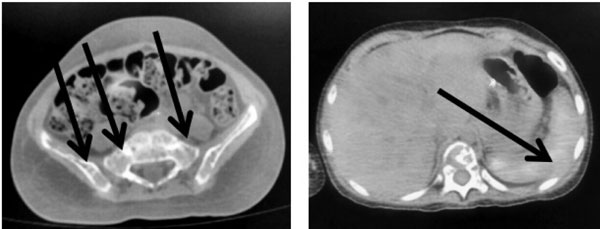 |
| (a) |
(b) |
|
Fig. 1 Fluorodeoxyglucose (FDG) PET
scan showing extensive lytic changes in skeleton (a) with
diffusely increased FDG uptake in spleen (b).
|
Radiographs showed extensive generalized bony lytic
lesions of skull (salt and pepper appearance), phalanges and spine with
vertebral collapse. Bone-scan showed metastatic calcification of stomach
and bilateral lung secondary to hypercalcemia. In the background of
extensive bony lytic lesions and suppressed PTH, malignancy-induced
hypercalcemia was considered. Complete blood count, peripheral smear,
lactate dehydrogenase (LDH), uric acid and alpha-fetoprotein (AFP) were
normal. Bone marrow aspiration and biopsy were normal. Parathyroid
related peptide (PTHrP) was also normal <0.8 pmol/L (<1.3). CECT
abdomen, chest and neck revealed diffuse geographic lytic lesions with
diffuse osteopenia (brown tumor) but no solid tumor was identified.
Fluorodeoxyglucose (FDG) PET scan showed extensive lytic changes
involving the entire visualized skeleton with diffusely increased FDG
uptake in the spleen suggestive of a lymphoproliferative disorder (Fig.
1a, 1b). A repeat bone marrow aspiration and biopsy revealed
inconclusive small round cell tumor. Subsequently, a CT-guided bone
marrow biopsy was done from iliac crest lesions, which showed 54%
malignant cells consistent with round cell tumor. Immuno-histochemistry
was positive for PAX5 (B cell marker), T’dt (precursor of lymphocytes),
MIC-2, and CD20 (focal) markers and negative for CD3 (T cell marker), CD
56, Chromogranin (neuroendocrine marker), which confirmed the diagnosis
of B-cell ALL. He was started on standard-risk induction chemotherapy
for ALL (vincristine, L-asparaginase, prednisolone and intra-thecal
methotrexate) with which remission was achieved.
In summary, our case presented with severe
hypercalcemia, high PTH and parathyroid adenoma on USG neck, pointing
towards a diagnosis of primary hyperparathyroidism (PHPT). Occurrence of
bony pains and the characteristic salt and pepper appearance of skull in
our patient were also consistent with PHPT. However, multiple extensive
bony lytic lesions are very rare in PHPT [2]. Serum PTH was found to be
suppressed on repeat testing pointing to the possibility of malignancy
in the absence of other clinical, haematological or biochemical
evidence. Our case report highlights the importance of repeating hormone
assays (PTH) when the clinical picture is inconsistent and the
limitations of a single rephine biopsy in detecting occult lymphoblastic
malignancies.
Hypercalcemia of malignancy is very rare in children
and usually seen in ALL, rhabdomyosarcoma and less often in lymphoma,
hepatoblastoma, and neuroblastoma [3]. Hypercalcemia in solid tumor
presents late in the disease course and is more resistant to treatment,
unlike ALL where it may present early without other clinical
manifestations [4]. There are several mechanisms of malignancy-induced
hypercalcemia, such as PTHrP by malignant solid tumours and osteolytic
metastasis with local release of cytokines including osteoclast
activating factor or unregulated extrarenal production of 1,25(OH)2D
(in lymphoma and granulomatous diseases) [5]. In one study of 22 ALL
patients with hypercalcemia, half had elevated PTHrP thought to be
released from blast cells [6]. In our case, the PTHrP was normal and
hypercalcemia probably resulted from the extensive osteolytic lesions
due to local pro-duction of cytokines and possibly high 1,25(OH)2D
levels.
We conclude that severe hypercalcemia, extensive
generalized bony lytic lesions and suppressed PTH levels may point to an
underlying malignancy even in the absence of occult features which
should be ruled out by appropriate investigations.
Contributors: KR: reviewed the literature and
drafted the manuscript; RS,MJ,VJ: critically reviewed the manuscript.
All authors were involved in management of the case and approved the
final manuscript.
Funding: None; Competing interest: None
stated.
References
1. Reagan P, Pani A, Rosner MH. Approach to diagnosis
and treatment of hypercalcemia in a patient with malignancy. Am J kidney Dis.
2014;63:141-7.
2. De Manicor NA. Primary hyperparathyroidism: a case
study. J Perianesth Nurs. 2004;19:334-41.
3. Fisher MM, Misurac JM, Leiser JD, Walvoord EC.
Extreme hypercalcemia of malignancy in a pediatric patient: therapeutic
consideration. AACE Clinical Case Rep. 2015;1:e12-5.
4. Celil E, Ozdemir GN, Tuysuz G, Tastan Y, Cam H,
Celkan T. A child presenting with hypercalcemia. Turk Pediatri Arsivi.
2014;49:81-3.
5. Davies JH. A Practical Approach to hypercalcemia.
In: Allgrove J, Shaw NJ, editors. Calcium and Bone Disorders in Children
and Adolescents. Endocrine Development. Basel: Karger Publishers; 2009.
p. 93-114.
6. Inukai T, Hirose K, Inaba T, Kurosawa H, Hama
A, Inada H, et al. Hypercalcemia in childhood acute lymphoblastic
leukemia: frequent implication of parathyroid hormone-related peptide
and E2A-HLF from translocation 17;19. Leukemia. 2007; 21:288-96.
|
|
|
 |
|

