|
|
|
Indian Pediatr 2019;56:951-957 |
 |
Allergy
Testing – An Overview
|
|
Neeraj Gupta 1,
Poojan Agarwal2,
Anil Sachdev1 and
Dhiren Gupta1
From 1Division of Pediatric
Emergency, Critical Care, Pulmonology and Allergic Disorders, Department
of Pediatrics, Institute of Child Health, and 2Department
of Pathology, Sir Ganga Ram Hospital, Rajinder Nagar, Delhi, India.
Correspondence to: Dr Neeraj Gupta,
Consultant, Division of Pediatric Emergency, Critical Care, Pulmonology
and Allergic Disorders, Department of Pediatrics, Institute of Child
Health, Sir Ganga Ram Hospital, Rajinder Nagar, Delhi 110060, India.
Email: [email protected]
|
|
Childhood allergies pose huge economic burden and adverse effects on
quality of life. Serum IgE has been considered a surrogate allergy
marker for decades. Availability of several over-the-counter allergy
tests add to confusion of partially trained caregivers. The present
review focuses on current status of allergy testing in Indian scenario.
Various in-vitro and in-vivo diagnostic modalities are
available for allergy detection. Skin prick tests are useful for
aero-allergies whereas oral challenge tests are best for identifying
suspected food allergies. An allergy test should be individualized based
on clinical features, diagnostic efficacy, and cost-benefit analysis.
Keywords: Challenge test, Histamine,
Hypersensitivity, IgE, Patch test, Skin prick test.
|
|
A
llergy encompasses a wide spectrum of
manifestations affecting skin, respiratory and gastrointestinal systems.
Several genetic, environmental and socio-economic factors play an
important role in the diverse presentation. A rising trend of allergies
has been noted worldwide affecting physical, social and psychological
well-being and increasing economic burden and disability adjusted life
years [1,2]. It is estimated that about 20-30% of the Indian population
suffers from atleast one form of allergy, of which, rhinitis is most
common followed by asthma [3,4]. Scarce diagnostic facilities and
limited knowledge further add onto the disease burden. This review
focuses on various available modalities for allergy diagnosis and their
clinical relevance.
Terminology
As per World Allergy Organization (WAO) and European
Academy of Allergology and Clinical Immunology (EAACI), hypersensitivity
is defined as a state when there are objectively reproducible symptoms
or signs initiated by an exposure to a defined stimulus at a dose
tolerated by other persons [5]. Allergy is a chronic clinical condition
involving an abnormal immune reaction to an ordinarily harmless
allergen, commonly mediated by IgE production though other mechanisms
can play significant role [6]. An IgE-mediated allergy with personal or
familial tendency is ‘atopy’. Hyper-sensitivity reactions usually have
an immunological basis but all are not allergies. This understanding is
necessary as most of the available allergy tests can only detect
hypersensitivity in response to a particular object/allergen, which
requires clinical correlation to be labelled as allergy.
Types of Hypersensitivity Reactions
1. Type I (Immediate or IgE-mediated) –
Rapid immunologic reaction in a previously sensitized individual,
triggered by binding of an antigen to IgE antibodies on the surface
of mast cells [7].
2. Type II (Antibody-mediated
cytotoxic/cytolytic) – IgG- and IgM- mediated cellular damage
with complement and phagocyte involvement.
3. Type III (Immune complex mediated) –
Antigen-antibody immune complex deposition causing complement
activation and tissue damage.
4. Type IV (Cell-mediated or delayed
hypersensitivity) – Direct cell damage mediated by various
cytokines released by sensitized Th1 cells.
Most of the clinically relevant allergies are
mediated via type I hypersensitivity. Commonly used in vitro
tests detect free IgE, whereas bound IgE and mast cell degranulation can
be demonstrated by in vivo procedures (skin-prick or challenge
tests). Food allergies have various IgE (e.g., urticaria,
angioedema, asthma, rhinitis, anaphylaxis and oral allergy) and non-IgE
(e.g., dermatitis hereptiformis, Heiner’s syndrome,
procto-enterocolitis, enteropathy and Celiac disease) mediated
mechanisms; hence, IgE-based tests alone are insufficient for their
diagnosis [8]. Atopic dermatitis and eosinophilic disorders may have
both types of mechanisms.
Allergy Diagnosis
The key lies in detailed clinical history, relevant
physical examination and knowledge about local environment. Temporal
relationship of allergen exposure and onset of clinical features,
periodicity of symptoms (seasonal/perennial, diurnal),
aggravating/relieving factors, history of travel, pet or insect
exposure, number of affected body systems, familial atopy and
occupational history should be elicited. Table I enlists
common indoor allergens and associated risk factors. Attending
physician/allergist should be aware of local aeroallergens with seasonal
variation (Web Table I) and regional predominance (Web
Table II). Knowledge about regional pollen calendar combined
with clinical correlation can help in tailoring allergen panel for
individual patient.
TABLE I Common Indoor Allergens
|
Organism |
Allergen |
Location |
Risk factors |
|
Dust Mite |
Der p1 and Der f1 |
Carpet, bedding (mattress/pillow/ |
Humidity, older homes and absence of air- |
|
|
curtains) and upholstery |
conditioning |
|
Dog |
Can f1 and albumin |
Carpet and bedding; airborne |
Depends on the breed and/or animal |
|
Cat |
Fel d1 |
Carpet and bedding; airborne |
Cat allergen is universal |
|
Cockroach |
Bla g1-4 and Per a1 |
Kitchen and dining |
Humidity and open food sources |
|
Fungus |
Various |
Bathrooms and/or kitchens; areas of water damage |
Humidity, water damage, and leaky plumbing |
|
Der p: Dermatophagoides pteronyssinus; Der f:
Dermatophagoides farinae; Can f: Canis familiaris; Fel d: Felis
domesticus; Bla g: Blattella germanica; Per a: Periplaneta
americana.
|
Laboratory Tests
Tests should be carefully selected based on patient
history, environmental triggers and operational issues (cost,
anaphylaxis risk, time required). Tests may be targeted towards either
cause identification or functional and structural disability assessment.
Immunological Tests: For Cause Identification
In vitro tests
(i) Total IgE levels – A reaginic
antibody was discovered by Ishizaka (1966) and Johansson (1967) groups
independently and named as IgE by WHO in 1968 [6]. Serum total IgE
levels, a conglomerate of all specific IgE molecules, are neither
sensitive nor specific for allergy diagnosis [9]. Raised levels may be
documented in conditions like parasitic infestations, immunodeficiency
disorders (e.g., AIDS, hyper IgE syndromes etc.), Ebstein Barr
virus (EBV) infection, rheumatoid arthritis and smoking [10]. IgE
molecules produced against specific individual antigens are labelled as
serum specific IgE (sIgE). These were detected by Radio-Allergo-Sorbent
Test (RAST) using radiolabeled (I 125)
antihuman IgE molecules. Radio-isotopes have now been replaced by enzyme
conjugated antihuman IgE antibodies (Immunocap). The major pitfall of
sIgE estimation is false positivity with high total IgE levels (>300
kIU/L) due to non-specific binding to test allergens [11].
(ii) Component resolved diagnostics (CRD):
Epitopes on some allergens may have structural homology with others.
For example, allergenic epitopes on birch pollen share similar
structural characteristics to peanut and hazel nut (Web Table
III), which may be responsible for oral pruritus while eating fresh
nuts in a birch pollen allergic individual without any true nut allergy
(oral allergy syndrome). During testing with crude allergen extract for
either of them there could be false positive reaction to others due to
phenomenon of cross reactivity. To combat this problem, recombinant
allergens using a specific epitope are being manufactured to improve
diagnostic efficacy of in-vitro food allergen tests [12] and better
management of the patients [13]. The high-risk component allergenic
proteins for peanut (Ara h1, 2, 3, 9), hazelnut (Cor a8, 9, 14), walnut
(Jug r1, 2, 3), soya (Gly m5, 6), wheat (Tri a14, 19) and Rosacea fruits
(Pru p3, Mal d3) can be detected with CRD [13]. It is a promising tool
for improving the specificity in allergy testing though more studies are
required for its validation.
In vivo tests
(i) Skin prick test (SPT) – SPT
is an age-old technique first described by Charles Harrison Blackley
(1860s) in patients of ‘hay fever’. It detects bound IgE by replicating
a mini allergic reaction in the already sensitized host once the
allergens are delivered epicutaneously. SPT is considered "gold
standard" test for diagnosing IgE mediated allergic diseases [14].
SPT should be performed at a place well equipped with
resuscitation facilities. As, blood vessels and pain receptors are
located in deep dermis, SPT is pain-free and associated with minimal
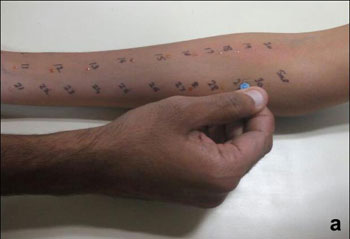
risk of bleeding or infection if performed appropriately (Fig.
1a) [15]. Crude extract may directly be pricked (Prick test)
followed by SPT, in case of non-availability of standard allergen
extract [16]. Selection of antigens should be based upon patient’s
clinical and environmental history, occupation and socio-economic
factors. House dust, house dust mite, relevant pollens (grass, tree or
weeds), fungus (Alternaria, Aspergillus), insects (Cockroach) and pet
animals (dog, cat, buffalo) dander are the commonest aeroallergens
prevalent in India whereas milk, egg, peanut, soya, wheat, tree nut,
fish and shell fish contribute to majority of food allergens [17,18].
Cockroach remains the predominant allergen among insects whereas common
occupational allergens are latex and chemicals. Honey bees are important
allergens in high risk groups like bee keepers. A patient with either
recurrent or persistent symptoms not adequately controlled by preventer
therapy should be tested with SPT. Patients with allergic rhinitis or
asthma having either persistent or moderate to severe symptoms as per
the ARIA and GINA guidelines should be subjected to allergy testing.
Patients on anti-histaminics and immunomodulators, on b-blockers, with
unhealthy skin condition, within 4 weeks of anaphylaxis and extremes of
age are not suitable for SPT. Clinical correlation of test results might
help in instituting specific allergen avoidance measures and targeted
immunotherapy in select cases.
 |
 |
|
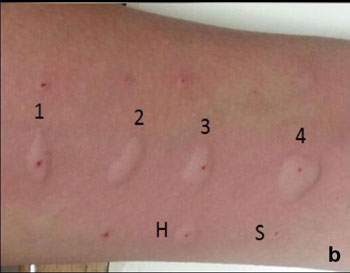
Fig. 1 Skin prick test: (a)
technique; (b) ‘Wheal’ and ‘Flare’ reactions after SPT to
different allergens (1,2,3,4).
|
Histamine and normal saline serve as positive and
negative controls respectively and read after 10 minutes and 15 minutes
[16]. Positive reaction is suggested by appearance of a wheal at the
prick site (Fig. 1b). The maximum diameter of the wheal is
measured and reaction interpreted in millimeters (mm) of wheal diameter
[19, 20]. Also, any pseudopod (an extra protuberance to the regular
circular shape) if observed, is separately mentioned. Positive control
should be at least 3 mm or more than negative control to establish test
validity. Any reaction with normal saline more than 3 mm should be
considered as baseline high reactivity of the skin, making test
conditions invalid. If positive and negative controls are within 3 mm of
each other, the test should be considered invalid. Any allergen showing
a wheal size of ³3
mm than the negative control should be considered positive indicator of
hypersensitivity. Any wheal size more than 8 mm suggests high positive
predictive value.
Factors influencing SPT
• Medications – Certain medications may
affect the results of SPT as mentioned in Table II
[16]. SPT reaction usually declines after 6 months to 3 years of
allergen immunotherapy.
• Age – SPT is currently practiced
beyond 6 months of age though no lower or upper age limit cutoff is
recommended [16]. Skin reactivity declines after 60 years.
• Test area – The mid and upper back are
33% more reactive than the lower back. The back as a whole is more
reactive (53%) than the forearm. An area approximately 5 cm away
from the wrist and 3 cm from the antecubital fossa, on the forearm
is usually used [16]. Left forearm is generally preferred.
• Distance between two pricks – A minimum
of 2 cm gap should be present between two adjacent test sites. This
is to prevent non-specific enhancement through nearby axon reflex
and also to avoid merging of wheals from strong reactions in the
nearby area.
• False positives – Skin conditions
(dermatographism, acute or chronic urticaria, cutaneous
mastocytosis), naturally occurring histamine in some allergen
extracts (insect venom, mold, foods), non-standard allergen
preparations (irritant reaction), cross-reactivity with homologous
proteins.
• False negatives – Recent (within 4
weeks) anaphylaxis, medications (Table II), technical
(low-potency extract, false technique), UV exposure.
TABLE II Drugs to be Stopped Before Skin Prick Test
|
Medication |
Period for withholding before test |
|
Antihistaminics (H1 blockers) |
48 hours |
|
Astemizole |
60 days |
|
Ketotifen |
5 days |
|
Tricyclic antidepressants |
2 weeks |
|
Short-term (topical/systemic) steroids |
No effects |
|
Long-term systemic steroids |
2 weeks |
|
Long-term topical steroids |
2-3 weeks |
The advantages of in vitro tests include no
effect of anti-histaminics or steroids, feasibility with any skin
condition and no risk of systemic reactions. Serum sIgE has better
specificity with higher positive predictive value for determining
aeroallergen (pollen and insects) sensitization [21]. SPT have an edge
over in-vitro tests in terms of better sensitivity with clinical
correlation, faster result (15-20 minutes), no interference with high
IgE levels and cost effectiveness [16]. Intradermal tests, with more
risk potential, are indicated only when SPT and/or sIgE results are
negative with relevant exposure history [22].
(ii) Patch test – It is based on
delayed (type IV) hypersensitivity. Patches with allergenic proteins are
applied on the upper back. An eczematous reaction usually occurs after
48-72 hours till as late as 7 days. Optimum reading time is day 2, 4 and
7 after patch application [23]. Test reactions are graded as erythema,
vesiculation or ulceration. TRUE test is one of the commercially
available patches with approximately 30 allergens. The most common
allergens are nickel sulfate, neomycin, myroxylon pereirae (balsam of
Peru), fragrance mix, thiomersal, sodium gold thiosulfate,
quaternium-15, formaldehyde, bacitracin and cobalt chloride [24]. Patch
test can be used at any age with negligible risk of anaphylaxis. It has
high specificity with very low sensitivity and is time consuming.
(iii) Nasal provocation test –
Increasing quantities of allergen extract are introduced in the anterior
part of inferior nasal turbinate to reciprocate the allergic reaction.
Due to higher chances of anaphylaxis and cumbersome technique, this is
not recommended.
(iv) Bronchial (Methacholine) challenge
test – Bronchoconstriction provoked by methacholine can be
quantified by spirometry. It should be done only in hospital setting
with availability of emergency facilities and is rarely performed
now-a-days.
(v) Oral food challenge test – Double
blind placebo control food challenge (DBPCFC) test is the gold standard
technique for detecting sensitivity to suspected food items. However,
due to practical difficulty in masking of food in a vehicle each time,
open label food challenge serves the needful [25]. When sIgE or SPT have
diminished substantially during the course of food allergy or in case of
false positive or negative skin or blood tests, oral challenge can be
used to confirm or rule out allergy.
(vi) Elimination trial test – In case
of true allergy the symptoms should disappear with food elimination and
reappear with its reintroduction [26].
Functional Assessment
Airway Hypersensitivity
Spirometry – Though gold standard for functional
assessment, effort dependence and requirement of patient cooperation
limits its practical utility in younger children and elderly. Evidence
of reversible bronchoconstriction is considered consistent with
diagnosis of asthma.
Impulse oscillometry – Determines airway
resistance, reactance and impedance using sound waves [27]. It can
detect peripheral airway obstruction, with minimal patient’s
cooperation, which may be missed by conventional spirometry [28].
Peak expiratory flow rate – Role limited to
monitor lung functions during domiciliary care. Diurnal variability in
lung functions is consistent with diagnosis of asthma.
Nasal Patency Assessment for Nasal Allergy
Rhino-manometry detects variable resistance
encountered to airstream in nasal passage. Peak nasal inspiratory
flowmeter is an economical, fast, portable, easy to use objective test
with good reproducibility.
Structural Assessment
Nasal endoscopy: It provides accurate assessment
of disease and anatomical variation in patients with symptoms of
rhinitis which may be important during surgical interventions. Apart
from revealing classical signs (watery nasal discharge and edematous
mucosa) it can help in detecting structural deformities (deviated
septum, septal spur, polyps, turbinate hypertrophy and stenotic or
accessary maxillary ostia), ruling out foreign bodies and collecting
samples (for cyto-, microbio- and histopatho-logical examination).
Gastrointestinal (GI) endoscopy: It may be
helpful during diagnosis (esophageal edema, furrows, exudates, transient
rings and diffuse narrowing) and management (stricture dilatation) in
eosinophilic esophagitis (EoE). Endoscopic guided GI tract biopsy may
provide a vital clue for eosinophilic inflammation.
Supportive Tests
Blood eosinophil levels: Hyper-eosinophilia (>450
cells/mL) may be present in Hodgkin lymphoma, Addison disease, allergies
(including asthma, eczema, allergic bronchopulmonary aspergillosis,
EoE), collagen vascular disorders, drug reactions, mastocytosis,
hyper-eosinophilic syndrome ( ³1500
eosinophils/mL) and infections (HIV, parasitic and fungal infections).
Nasal and sputum eosinophilia: It has been
documented in patients with allergic asthma.
Fractional Exhaled Nitric Oxide (FENO): FENO is a
good surrogate marker for eosinophilic airway inflammation and can
assist in treatment of refractory asthma cases.
Mast cell mediators: High histamine levels soon
after anaphylaxis is the best investigation but practically impossible
due to short half-life (2-3 minutes). Serum tryptase levels peaks
between 15 to 20 minutes (half-life of 2.5 hours.) Blood samples are
collected at 1, 2, 3, 6, 12 and 24-hours post-reaction to document both
rise and fall. A peak concentration of >50 mg/L with relevant symptoms
and documented fall during convalescence is consistent with IgE-mediated
anaphylaxis [29].
Pitfalls of Allergy Testing
The main limitation of allergy testing is that it
detects only IgE-mediated hypersensitivity state, which may not be
clinically relevant. sIgE may be falsely positive with high total IgE
levels. SPT might be falsely negative after certain medications and
anaphylaxis.
Conclusion
Serum and skin tests help in detecting IgE-mediated
hypersensitivities, which require clinical correlation. While choosing
an allergy test panel, one needs to be vigilant about relevant allergens
as per exposure history and cost to benefit ratio for an individual
patient. SPT is a reliable, cost and time effective modality when
performed using standard extracts. Patch test may be useful in delayed
hypersensitivity reactions. Component resolved diagnostics, a futuristic
tool, might be helpful in cross reactive food allergies. ‘Less is more’
should be the dictum, regarding number of allergens to be tested.
Identification of responsible allergens helps in specific allergen
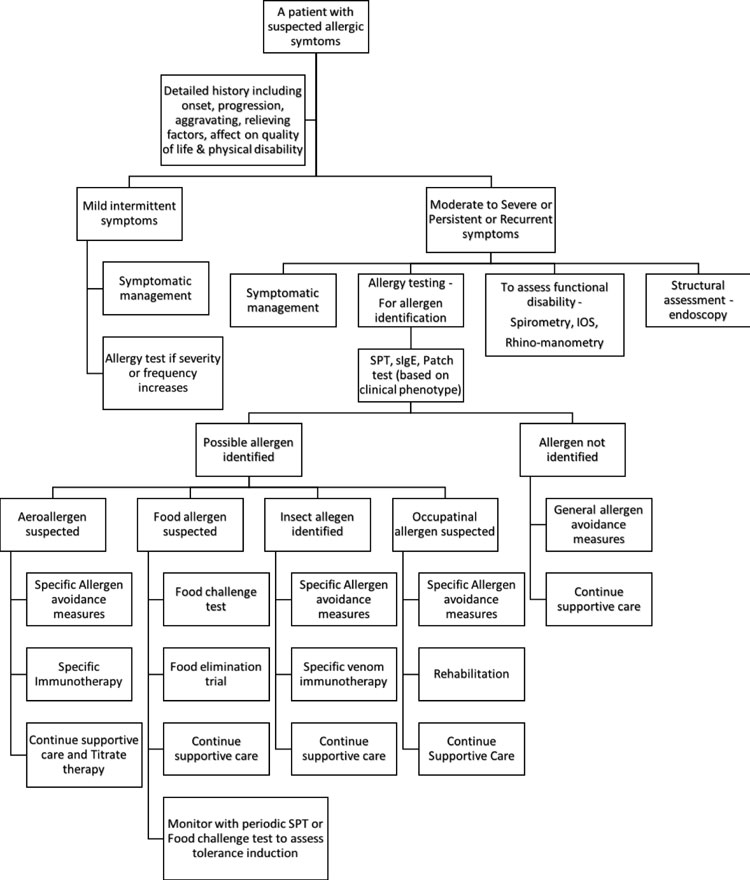
avoidance measures and targeted immuno-therapy. Fig. 2
gives an algorithmic approach to an allergic patient.
 |
|
Fig. 2 Approach to an allergic
patient.
|
Contributors: NG: conceptualized and
designed the original manuscript, wrote the initial draft and revised it
critically; PG: helped in designing the initial draft by writing the
immunological part and revised the final manuscript; AS,DG: helped in
designing the original manuscript by writing structural and functional
allergy assessment section and revised it critically for important
intellect. All authors approved the final version of the manuscript and
agree to be accountable for all aspects of the work.
Funding: None; Competing interest: None
stated.
|
Key Messages
• Blood tests target free IgE while skin
tests and challenge tests mimic natural reactions by targeting
bound IgE and mast cell degranulation.
• Skin prick test is considered the gold
standard for aero-allergen identification, while challenge tests
take precedence in suspected food allergies.
• Allergy testing is recommended in
moderate-severe or persistent or recurrent symptoms.
• Clinical symptoms, local flora and occupational exposure
should be kept in mind while selecting an allergy panel.
|
References
1. Pawankar R. Allergic diseases and asthma: A global
public health concern and a call to action. World Allergy Organ J.
2014;7:12.
2. Antolin-Amerigo D, Manso L, Caminati M, de la Hoz
Caballer B, Cerecedo I, Muriel A, et al. Quality of life in
patients with food allergy. Clin Mol Allergy. 2016;14:4.
3. Prasad R, Kumar R. Allergy Situation in India:
what is being done? Indian J Chest Dis Allied Sci. 2013;55:7-8.
4. Chandrika D. Allergic rhinitis in India: an
overview. Int J Otorhinolaryngol Head Neck Surg. 2017;3:1-6.
5. Pawankar R, Holgate ST, Canonica GW, Lockey RF.
White Book on Allergy. Milwaukee, Winconsin, United State of America.
World Allergy Organization (WAO); 2011.
6. Igea JM. The history of the idea of allergy.
Allergy. 2013;68:966-73.
7. Eastman J, Gupta R, Jose J. Immune Mechanisms.
In: Lee G, Stukus D, Yu Joyce, editors. Review for the Allergy &
Immunology Boards. 3rd ed. Arlington Heights, United States of America.
American College of Allergy, Asthma & Immunology (ACAAI); 2016.p.45-6.
8. Sicherer SH, Sampson HA. Food allergy: A review
and update on epidemiology, pathogenesis, diagnosis, prevention and
management. J Allergy Clin Immunol. 2018;141:41-58.
9. Abraham JT, Barcena MA, Bjelac JA, Pazheri FP.
Specific Diagnostic Modalities. In: Lee G, Stukus D, Yu Joyce,
editors. Review for the Allergy & Immunology Boards, 3rd ed. Arlington
Heights, United States of America. American College of Allergy, Asthma &
Immunology (ACAAI); 2016.p.417-500.
10. Schimke LF, Sawalle-Belohradsky J, Roesler J,
Wollenberg A, Rack A, Borte M, et al. Diagnostic approach to the
hyper-IgE syndromes: immunologic and clinical key findings to
differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin
Immunol. 2010;126:611-7.
11. de Vos G, Nazari R, Ferastraoaru D, Parikh P,
Geliebter R, Pichardo Y, et al. Discordance between aeroallergen
specific serum IgE and skin testing in children younger than 4 years.
Ann Allergy Asthma Immunol. 2013;110:438-43.
12. Treudler R, Simon JC. Overview of component
resolved diagnostics. Curr Allergy Asthma Rep. 2013;13:110-7.
13. Borres MP, Maruyama N, Sato S, Ebisawa M. Recent
advances in component resolved diagnosis in food allergy. Allergol Int.
2016;65:378-87.
14. Bernstein IL, Li JT, Bernstein DI, Hamilton R,
Spector SL, Tan R, et al. Allergy diagnostic testing: an updated
practice parameter. Ann Allergy Asthma Immunol. 2008;100: S1-148.
15. Kowalski ML, Ansotegui I, Aberer W, Al-Ahmad M,
Akdis M, Ballmer-Weber BK, et al. Risk and Safety Requirements
for Diagnostic and Therapeutic Procedures in Allergology: World Allergy
Organization Statement. World Allergy Organ J. 2016;9:33.
16. Smith W. Skin Prick Testing for the Diagnosis of
Allergic Disease – A Manual for Practitioners. Australasian Society of
Clinical Immunology and Allergy (ASCIA). Available from:
www.allergy.org.au/images/stories/pospapers/ASCIA_SPT_Manual_March_2016.pdf.
Accessed September 26, 2019.
17. Bhattacharya K. Sircar G, Dasgupta A, Gupta
Bhattacharya S. Spectrum of allergens and allergen biology in India. Int
Arch Allergy Immunol. 2018;177:219-37.
18. Comberiati P, Cipriani F, Schwarz A, Posa D, Host
C, Peroni DG. Diagnosis and treatment of pediatric food allergy: an
update. Ital J Pediatr. 2015;41:13.
19. van der Valk JP, Gerth van Wijk R, Hoorn E,
Groenendijk L, Groenendijk IM, de Jong NW. Measurement and
interpretation of skin prick test results. Clin Transl Allergy.
2016;6:8.
20. Haahtela T, Burbach GJ, Bachert C, Bindslev-Jensen
C, Bonini S, Bousquet J, et al. Clinical relevance is associated
with allergen-specific wheal size in skin prick testing. Clin Exp
Allergy. 2014;44:407-16.
21. Alimuddin S, Rengganis I, Rumende CM, Setiati S.
Comparison of specific immunoglobulin E with the skin prick test in the
diagnosis of house dust mites and cockroach sensitization in patients
with asthma and/or allergic rhinitis. Acta Med Indones. 2018;50:125-31.
22. Ferastraoaru D, Shtessel M, Lobell E, Hudes G,
Rosenstreich D, de Vos G. Diagnosing environmental allergies: Comparison
of skin-prick, intradermal, and serum specific immunoglobulin E testing.
Allergy Rhinol (Providence). 2017;8:53-62.
23. Bourke J, Coulson I, English J. Guidelines for
the management of contact dermatitis: an update. Br J Dermatol.
2009;160:946-54.
24. AlSelahi EM, Cooke AJ, Kempe E, Schiffman AB,
Sokol K, Virani S. Hypersensitivity disorders. In: Lee G, Stukus
D, Yu Joyce, editors. Review for the allergy & immunology boards. 3rd
ed. Arlington Heights, United States of America. American College of
Allergy, Asthma & Immunology (ACAAI); 2016.p.129-202.
25. Sampson HA, Aceves S, Bock SA, James J, Jones S,
Lang D, et al. Food allergy: A practice parameter update-2014. J
Allergy Clin Immunol. 2014;134:1016-25.
26. Caffarelli C, Baldi F, Bendandi B, Calzone L,
Marani M, Pasquinelli P, et al. Cow’s milk protein allergy in
children: A practical guide. Ital J Pediatr. 2010;36:5.
27. Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi
S, Galant SP, George SC. Relating small airways to asthma control by
using impulse oscillometry in children. J Allergy Clin Immunol.
2012;129:671-78.
28. Dos Santos K, Fausto LL, Camargos PAM, Kviecinski
MR, da Silva J. Impulse oscillometry in the assessment of asthmatic
children and adolescents: From a narrative to a systematic review.
Paediatr Respir Rev. 2017;23:61-7.
29. Sheldon J, Philips B. Laboratory investigation of anaphylaxis:
Not as easy as it seems. Anaesthesia. 2015;70:1-5.
|
|
|
 |
|

