|
|
|
Indian Pediatr 2019;56: 939-950 |
 |
Hexavalent Vaccines in India: Current Status
|
|
Amar Jeet Chitkara 1,
Raunak Parikh2,
Attila Mihalyi3
and Shafi Kolhapure2
From 1Department of
Pediatrics, Max Superspeciality Hospital, Delhi,
India; 2Glaxo Smith Kline Biologicals SA,
Mumbai, India; and 3Glaxo Smith Kline Biologicals
SA, Wavre, Belgium.
Correspondence to: Dr Raunak
Parikh, GSK, Dr. Annie Besant Road, Mumbai 400 030,
Maharashtra, India.
Email:
[email protected]
|
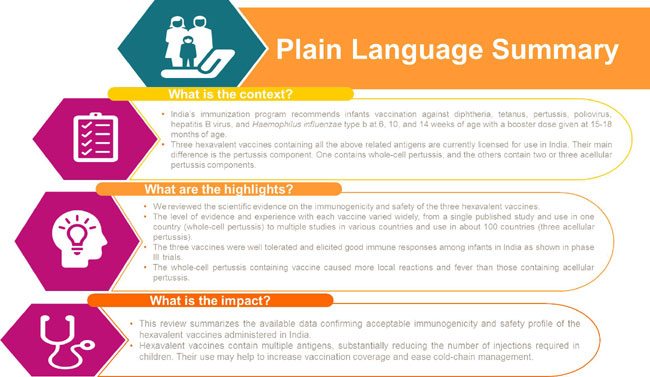
Hexavalent vaccines containing diphtheria,
tetanus, pertussis, Haemophilus influenzae
type b, poliomyelitis, and hepatitis B virus
antigens have the potential to be used for the
primary series in India (6, 10, 14 weeks of age)
and the toddler booster dose. Three hexavalent
vaccines are available in India: DTwP-Hib/HepB-IPV
(wP-hexa), DTaP-IPV-HB-PRP~T(2aP-hexa), and DTaP-HBV-IPV/Hib
(3aP-hexa). In the three published phase-3
Indian studies, pertussis vaccine response
rates 1 month after a 6-10-14-week primary
series were 68.4-75.7% for wP-hexa, 93.8-99.3%
for 2aP-hexa, and 97.0-100% for 3aP-hexa;
seroprotection rates for the other five antigens
were 88.2-100%, 49.6-100%, and 98.6-100%,
respectively. Studies outside India show: good
immunogenicity/safety after boosting dosing;
immune persistence to age 4.5 years (2aP-hexa),
7-9 years (3aP-hexa) (all antigens), and 9-10
and 14-15 years, respectively (hepatitis B); and
successful co-administration with other
vaccines. Hexavalent vaccines could reduce the
number of injections, simplify vaccination
schedules, and improve compliance.
Keywords: Combination vaccines,
Acellular Vaccine, Immunization, Pertussis.
|
|
C ombination
vaccines help to protect against different diseases,
offer a solution to the problem of increasing
numbers of injections during the first two years of
life, and can help simplify vaccination schedules
[1,2]. The United States (US) Advisory Committee on
Immunization Practices (ACIP) has recommended that
combination vaccines are preferred over lower-valent
vaccines provided they are licensed and indicated
[2]. However, they must not be less immunogenic,
less efficacious, or more reactogenic than lower-valent
vaccines [1]. Although combination vaccines can be
more expensive than their component vaccines, they
may offer better economic value if direct and
indirect costs of extra injections, delayed or
missed vaccinations, and additional handling and
storage requirements are considered [2].
A combined vaccine against
diphtheria, tetanus, and pertussis (DTP), which
contained whole-cell pertussis (DTwP), was
introduced in 1948 [1]. Its acellular pertussis
equivalent (DTaP) became available in the early
1990s [1]. DTP vaccines have since been combined
with other vaccine antigens (Haemophilus
influenzae type b [Hib], poliomyelitis,
hepatitis B [HepB] virus [HBV]) to make pentavalent
vaccines such as DPT-HBV-Hib and DTaP-IPV/Hib.
Vaccines containing antigens against all six
diseases have also been manufactured. The hexavalent
vaccines offer the general benefits of higher valent
combination vaccines for children, parents and
healthcare providers [3-5]. This review discusses
the evidence related to the use of the hexavalent
vaccines that are currently licensed in India (Fig.1).
This narrative review was done following a
comprehensive search of electronic databases in
English and was undertaken with broad overview of
topic-related search in Pub Med, and Embase for the
period 2000-2018 with keywords "hexavalent
vaccines", "DTP", "immunogenicity", "pertussis", and
"India" used alone or in combination. Additional
relevant information from prescribing information
(and related referred studies within it), government
websites, and World Health Organization (WHO)
website were also considered.
 |
|
Fig. 1 The study
in context.
|
Vaccination Schedules in India
During the first two years of
life, the Universal Immunization Program (UIP) in
India recommends vaccination against the six
diseases covered by the hexavalent vaccines with:
oral poliovirus (OPV) and HBV vaccines at birth;
pentavalent DPT-HBV-Hib plus OPV at age 6, 10, and
14 weeks; fractional doses (1/5 full dose,
intradermal route) of inactivated polio vaccine
(IPV) at 6 and 14 weeks; and boosters of OPV and DTP
at 16-24 months [6].
For private practitioners, the
Indian Academy of Pediatrics (IAP) recommends OPV
and HBV vaccines at birth; DTP, HBV, and Hib (or
pentavalent vaccine), and intramuscular IPV at age
6-10-14 weeks; and DTP, Hib, and IPV at 16-18 months
[7].
Polio Component
The most notable difference
between the UIP [6] and IAP [7] schedules is that
they recommend fractional and intramuscular IPV,
respectively. This relates to recent WHO
recommendations on immunization against
poliomyelitis [8]. Rarely, the Sabin poliovirus
strains in OPV can cause vaccine-associated
paralytic polio; they can also mutate to circulating
vaccine-derived polio virus (cVDPV), which can cause
outbreaks [9]. It was therefore decided to phase out
use of OPV and replace them with IPV [10]. As wild
type 2 poliovirus has been eradicated worldwide and
90% of circulating vaccine-derived polio virus cases
were caused by Sabin type 2 poliovirus [9], the
first step in this transfer was to replace trivalent
OPV which contains types 1, 2, and 3 with
bivalent OPV that contains types 1 and 3. However,
to provide protection against type 2, at least one
full dose of IPV (which contains all three types)
also needs to be administered [10,11].
Due to the resultant worldwide
requirement for IPV, there are some problems with
supply [12]. One way to overcome this issue is to
use two fractional intradermal IPV doses, which
contain one-fifth of the dose [10], instead of one
full intramuscular dose. The worldwide switch to
bivalent OPV took place in April 2016 and fractional
IPV was introduced into the UIP [6,10,13]. IAP has
already taken the next step and switched completely
to three full doses of IPV in the primary series,
with a booster dose in the second year of life
whenever possible [7]. This IPV schedule is
consistent with countries that have withdrawn OPV
and instead use IPV as a 2- or 3-dose primary series
in infancy, with 0 or 1 booster dose at 6-24 months,
and 0 or 1 preschool booster dose [11].
Pertussis Component
Vaccines containing whole-cell
pertussis (wP) were introduced first [14], and their
efficacy varied between 46% and 92% (pooled 78%)
[15]. However, wP-containing vaccines were
associated with high rates of swelling, induration,
fever, and prolonged crying [15]. Due to these
reactogenicity issues, many countries switched to
vaccines containing acellular pertussis (aP)
components [14]. Although aP-containing vaccines had
slightly lower pooled efficacy (73%), their efficacy
seemed to be more consistent (67-84%) and
reactogenicity was lower [15]. While wP-containing
vaccines have been used in national programs in
several countries including India where they have
had an acceptable safety profile, historically
aP-containing vaccines have been demonstrated to be
less reactogenic than wP-containing vaccines. WHO
has reported observed rates of vaccine reactions of
DTP vaccines, with wP-containing vaccines associated
with 2-6-fold increases in fever
³38.4°C
(15.9% vs. 3.7%), redness
³20mm
(16.4% vs. 3.3%), swelling
³20
mm (22.4% vs. 4.2%), moderate-to-severe pain
(39.9% vs. 6.9%), anorexia (35% vs.
21.7%), and moderate-to-severe fussiness (41.5%
vs. 17.1%)%) compared with aP-containing
vaccines [16]. wP-containing vaccines are also
associated with more serious adverse events than
aP-containing vaccines: persistent screaming (3.5%
vs. 0-0.2%), hyporesponsive hypotonic
episodes (57-250 vs. 14-62 per 100,000),
seizures (6 vs. 0.5 per 100,000), and
encephalopathy (0.3-5.3 per 1,000,000 vs. no
documented risk) [16].
aP-containing vaccines can
contain 1, 2, 3, or 5 of the following antigens:
pertussis toxin (PT), filamentous hemagglutinin
(FHA), pertactin (PRN), and fimbriae types 2 and 3
(FIM 2 and 3) [14]. A 2014 review reported that
aP-containing vaccines with
³3
components had higher efficacy against typical
whooping cough than those containing 1 or 2
components (84-85% vs. 59-78%) [17]. However,
other evidence has suggested that efficacy may not
simply be related to the number of components [18].
Animal studies using the baboon
model suggest that wP-containing vaccines
predominantly elicit a Th17/Th1 response which may
provide a longer lasting protection than the Th1/Th2
response elicited by aP-containing vaccines; also
the predominant Th2 (but lower Th1 and Th17)
responses seen with aP-containing vaccines may be
less effective in clearing B. pertussis and
preventing transmission [18]. However, it is
important to note that in the baboon studies, the
animals were vaccinated with DTaP vaccines without
additional antigens such as IPV. This is relevant
for the immune response elicited by the hexavalent
vaccines because it is described in the literature
that the ssRNA of the inactivated polio vaccine has
an adjuvant effect via TLR7 and TLR8 [19,20].
In a recent mouse model, it was also shown that
addition of a TLR7 agonist to an alum-adjuvanted aP
vaccine converts it from a Th2-inducing vaccine to a
more Th1/Th17-inducing vaccine with higher
protective capacity, equivalent to or greater than
that of a wP vaccine in a murine model [21]. In view
of this, presence or absence of IPV in combination
vaccines may have an impact on the immune response
and the protective efficacy of the vaccine against
pertussis.In some countries that switched from wP-
to aP-containing vaccines, there was a resurgence in
pertussis several years after the switch [18]. This
may have been due to shorter duration of protection
and lower impact of transmission seen with
aP-containing vaccination. However, pertussis
resurgence is not universal and the incidence of
pertussis already increased in some countries before
the switch to aP vaccines [22,23]. Following
evaluation of data from 19 middle/high-income
countries, WHO concluded that there was "no evidence
of a widespread resurgence" of pertussis [18,24].
Increases in pertussis cases were mostly attributed
to naturally occurring cyclic patterns [18,24].
Other factors that could have contributed to the
increase in cases included higher pertussis
awareness, improved surveillance, and better
diagnostic techniques [18,25]. It is noteworthy that
no country that switched from wP to aP is
considering reverting to wP, probably because this
could result in poor acceptance, lower uptake, and
increased disease burden even if wP vaccines could
potentially offer higher efficacy and longer
protection [26]. Further, examination of pertussis
incidence trends from 20 countries that switched
from wP- to aP-containing vaccines did not indicate
a correlation between switch date and pertussis
incidence [27].
In 2013, the IAP recommended
wP-containing vaccines for the primary series [28],
but in the 2018 revision, it stated that either DTwP
or DTaP can be used, with the primary aim of
increasing vaccination coverage [7]. When
vaccinating healthy children in private practice,
both benefits and risks must be considered when
deciding whether to use aP- or wP-containing
vaccines. While both are effective in preventing
pertussis, both are associated with waning immunity
and require booster doses. Regarding safety,
aP-containing vaccines have been associated with a
more favorable safety profile than wP-containing
vaccines [16,29].
Potential Scheduling of
Hexavalent Vaccines in India
Hexavalent vaccines provide the
required antigens for the primary series (6-10-14
weeks), but can also be considered for 16-18-month
booster vaccination according to the IAP schedule,
involving an additional HBV dose [30]. This would
likely be acceptable as the US ACIP has recommended
that administering extra antigen(s) in a combination
vaccine "is often permissible if doing so will
reduce the number of injections required" and "an
extra dose of Hib or HepB vaccine may be
administered as part of a combination vaccine to
complete a vaccination series" [2]. Further, five
doses of HBV vaccine (birth, three primary, one
booster) has been assessed in trials with hexavalent
vaccines [31,32], and this number of anti-HBV doses
did not appear to affect the vaccine safety
profiles. In both trials, there were multi-fold
increases in hepatitis B surface antigen (HBs)
titers one month after booster vaccination compared
to one month after primary vaccinations, regardless
of whether a birth dose of HBV vaccine had been
administered or not.
Hexavalent Vaccines Efficacy Data
From India
Three IPV-containing hexavalent
vaccines are available in India: DTwP-Hib/HepB-IPV
(Panacea Biotec [33]), DTaP-IPV-HB-PRP~T (Sanofi
Pasteur [34]), and DTaP-HBV-IPV/Hib (GSK [35,36]) (Table
I).The main difference in their composition is
that DTwP-Hib/HepB-IPV contains a wP component [33],
DTaP-IPV-HB-PRP~T contains two aP components [34],
and DTaP-HBV-IPV/Hib contains three aP components
[35,36]. We will therefore refer to them as wP-hexa,
2aP-hexa, and 3aP-hexa, respectively. wP-hexa has
been available since 2017 and is only available in
India. 2aP-hexa was launched in 2013 and has been
available in India since 2016. 3aP-hexa was launched
in 2000 and has been available in India since 2018.
TABLE I Overview of Hexavalent Vaccines Currently Available in India
|
Vaccine |
DTwP-Hib/HepB-IPV
|
DTaP-IPV-HB-PRP~T
|
DTaP-HBV-IPV/Hib
|
|
(wP-hexa) [33] |
(2aP-hexa) [34] |
(3aP-hexa) [35,36] |
|
Components |
|
|
|
|
Diphtheria |
DT ³30 IU |
DT ³20 IU |
DT ³30 IU |
|
Tetanus |
TT ³60 IU |
TT ³40 IU |
TT ³40 IU |
|
Pertussis |
Inactivated whole-cell |
PT 25 µg |
PT 25 µg |
|
B. pertussis ³4 IU |
FHA 25 µg |
FHA 25 µg |
|
|
|
PRN 8 µg |
|
Hepatitis B |
HBs 10 µg |
HBs 10 µg |
HBs 10 µg |
|
Poliovirus |
Type 1* 40 DU |
Type 1* 40 DU |
Type 1* 40 DU |
|
Type 2# 8 DU |
Type 2# 8 DU |
Type 2# 8 DU |
|
Type 3$ 32 DU |
Type 3$ 32 DU |
Type 3$ 32 DU |
|
Hib |
Hib polysaccharide (PRP) |
Hib polysaccharide (PRP) |
Hib polysaccharide (PRP) |
|
10 µg (TT carrier) |
12 µg (TT carrier) |
10 µg (TT carrier) |
|
Primary series |
6-10-14 wk |
6-10-14 wk; |
6-10-14 wk; |
|
dosing schedules tested |
|
2-3-4 or 2-4-6 mo; |
2-3-4 or 2-4-6 or |
|
|
3 and 5 mo |
3-4-5 mo; 2 and 4 or |
|
|
|
3 and 5 mo |
|
6-10-14-wk schedule tested |
India [37] |
India [38], South Africa [42] |
India [39], Philippines [31] |
|
*Mahoney strain; #MEF-1 strain; $Saukett
strain; DT: diphtheria toxoid; DU: D-antigen
unit; FHA: filamentous hemagglutinin; HBs:
hepatitis B surface antigen; Hib:
Haemophilus influenzae type b; PRN:
pertactin; PRP: polyribosylribitol
phosphate; PT: pertussis toxoid; TT: tetanus
toxoid. |
Published results are available
from one phase 3 wP-hexa study, conducted in India
[37], in which it was administered as a primary
series at 6-10-14 weeks. 2aP-hexa and 3aP-hexa have
been studied in a number of different dosing
schedules, including 2- or 3-dose primary series (Table
I) and as a booster during the second year of
life [34-36] in several countries.
Primary Doses
One phase 3 study in Indian
infants for each of the three hexavalent vaccines
has been published [37-39] (Table II).
In the wP-hexa study, 284 healthy Indian infants
were randomized to wP-hexa or pentavalent
DTwP-HBV-Hib plus IPV at 6-10-14 weeks [37]; it is
unclear whether all infants had received birth doses
of HBV and OPV vaccines. In the 2aP-hexa study, 177
healthy Indian infants who had received birth doses
of HBV and OPV vaccines received 2aP-hexa at 6-10-14
weeks [38]. In the 3aP-hexa study, 224 Indian
infants who had received birth doses of HBV and OPV
vaccines were randomized 1:1 to 3aP-hexa at 6-10-14
weeks or 2-4-6 months [39].
TABLE II Seroprotection/Vaccine Response Rates One month after Primary Vaccination with Three Doses of Hexavalent Vaccine
(at 6-10-14 weeks) in Indian Infants Who Had Received a Birth Dose of HBV Vaccinea
|
wP-hexa [37]
|
2aP-hexa [38] |
3aP-hexa [39,40] |
|
wP-hexa |
Control arm |
2aP-hexa (n=156) |
3aP-hexa (n=105) |
Control arm |
|
(n=136) |
(n=136) |
|
6-10-14 wk group |
(n=106) |
|
|
wP-penta+Polio |
|
|
3aP-hexa |
|
|
|
|
|
2-4-6 mo group |
|
Seroprotection |
|
Anti-diphtheria (≥0.01
IU/mL)b |
NR |
NR |
99.3 (95.9-100) |
NR |
NR |
|
Anti-diphtheria (≥0.1
IU/mL)b |
94.9 (89.7-97.9) |
95.6 (90.6-98.4) |
49.6 (40.9-58.4) |
100 (96.5-100) |
100 (96.6-100) |
|
Anti-tetanus (≥0.01
IU/mL)c |
NR |
NR |
100 (97.3-100) |
NR |
NR |
|
Anti-tetanus (≥0.1
IU/mL)c |
100 (97.3-100) |
100 (97.3-100) |
NR |
100 (96.5-100) |
100 (96.6-100) |
|
Anti-HBs (≥10
mIU/mL) |
97.8 (93.7-99.5) |
97.1 (92.6-99.2) |
100 (97.6-100) |
100 (96.4-100) |
99.0 (94.8-100) |
|
Anti-Polio type 1 (≥1:8)
|
89.7 (83.3-94.3) |
91.9 (86.0-95.9) |
100 (97.5-100) |
100 (96.3-100) |
100 (96.3-100) |
|
Anti-Polio type 2 (≥1:8) |
93.4 (87.8-96.9) |
94.1 (88.7-97.4) |
100 (97.5-100) |
100 (95.3-100) |
100 (95.9-100) |
|
Anti-Polio type 3 (≥1:8) |
88.2 (81.6-93.1) |
90.4 (84.2-94.8) |
100 (97.5-100) |
98.6 (92.7-100) |
100 (95.4-100) |
|
Anti-PRP (≥0.15
µg/mL)d |
100 (97.3-100) |
100 (97.3-100) |
100 (97.7-100) |
99.0 (94.8-100) |
99.1 (94.9-100) |
|
Anti-PRP (≥1 µg/mL)d |
92.7 (86.9-96.4) |
89.0 (82.5-93.7) |
93.6 (88.5-96.9) |
NR |
NR |
|
Vaccine response (for pertussis) |
|
|
|
|
|
|
Anti-PTe,f,g |
68.4 (59.9-76.1)e |
66.2 (57.6-74.1) |
93.8 (88.6-97.1)f |
100 (96.5-100)g |
99.0 (94.8-100) |
|
Anti-FHAf.g |
NR |
NR |
99.3 (96.3-100)f |
97.0 (91.6-99.4)g |
98.0 (93.1-99.8) |
|
Anti-PRNg |
NR |
NR |
NR |
99.0 (94.8-100)g |
99.0 (94.8-100) |
|
Pertussis IgGe |
75.7 (67.6-82.7)e |
72.8 (64.5-80.1) |
NR |
NR |
NR |
|
Data are % (95% CI). *Pentavac SD (Serum
Institute of India Ltd) and Imovax Polio (Sanofi
Pasteur India Pvt. Ltd); aIt is not clear
whether all infants in the wP-hexa study
[37] received a birth dose of HBV vaccine;
bWHO-defined levels for seroprotection
against diphtheria are 0.01 IU/mL (some
protection) and 0.1 IU/mL (full protection)
using a toxin neutralization test [41]. The
wP-hexa and 3aP-hexa studies used ELISA [37,
39]; the 2aP-hexa study used a
neutralization assay [38]; cWHO-defined
levels for seroprotection against tetanus
are 0.01 IU/mL (neutralization test or
modified ELISA) and 0.1-0.2 IU/mL (standard
ELISA) [67]. The wP-hexa study used a
specific ELISA [38]; the 2aP-hexa study
ELISA [39]; the 3aP-hexa study standard
ELISA [38]; dWHO-defined levels for
seroprotection against Hib are 0.15 µg/mL
(short-term protection) and 1 µg/mL
(long-term protection) [68]; eIf
seronegative pre-vaccination: ³100 µg/mL for
anti-PT or ³18 IU/mL for pertussis IgG; if
seropositive pre-vaccination: ³4-fold
increase in antibody titer level; fIf
pre-vaccination concentrations <4 × LLOQ: ³4
× LLOQ of the assay (2 IU/mL); if
pre-vaccination concentrations ³4 × LLOQ,
³pre-vaccination concentration; gIf
seronegative pre-vaccination: ³5 EL.U/mL; if
seropositive pre-vaccination: ³1-fold
increase in antibody concentration; CI:
confidence interval; ELISA: enzyme-linked
immunosorbent assay; FHA: filamentous
hemagglutinin; HBs: hepatitis B surface
antigen; HBV: hepatitis B virus; Hib:
Haemophilus influenzae type b; IgG:
immunoglobulin G; LLOQ: lower limit of
quantification; NR: not reported; PRN:
pertactin; PRP: polyribosylribitol
phosphate; PT: pertussis toxoid. |
TABLE II Seroprotection/Vaccine Response Rates One month after Primary Vaccination with Three Doses of
Hexavalent Vaccine (at 6-10-14 weeks) in Indian Infants Who Had Received a Birth Dose of HBV Vaccinea
|
wP-hexa [37]
|
2aP-hexa [38] |
3aP-hexa [39,40] |
|
wP-hexa |
Control arm |
2aP-hexa (n=156) |
3aP-hexa (n=105) |
Control arm |
|
(n=136) |
(n=136) |
|
6-10-14 wk group |
(n=106) |
|
|
wP-penta+Polio |
|
|
3aP-hexa |
|
|
|
|
|
|
2-4-6 mo group |
|
Seroprotection |
|
Anti-diphtheria (≥0.01
IU/mL)b |
NR |
NR |
99.3 (95.9-100) |
NR |
NR |
|
Anti-diphtheria (≥0.1
IU/mL)b |
94.9 (89.7-97.9) |
95.6 (90.6-98.4) |
49.6 (40.9-58.4) |
100 (96.5-100) |
100 (96.6-100) |
|
Anti-tetanus (≥0.01
IU/mL)c |
NR |
NR |
100 (97.3-100) |
NR |
NR |
|
Anti-tetanus (≥0.1
IU/mL)c |
100 (97.3-100) |
100 (97.3-100) |
NR |
100 (96.5-100) |
100 (96.6-100) |
|
Anti-HBs (≥10
mIU/mL) |
97.8 (93.7-99.5) |
97.1 (92.6-99.2) |
100 (97.6-100) |
100 (96.4-100) |
99.0 (94.8-100) |
|
Anti-Polio type 1 (≥1:8)
|
89.7 (83.3-94.3) |
91.9 (86.0-95.9) |
100 (97.5-100) |
100 (96.3-100) |
100 (96.3-100) |
|
Anti-Polio type 2 (≥1:8) |
93.4 (87.8-96.9) |
94.1 (88.7-97.4) |
100 (97.5-100) |
100 (95.3-100) |
100 (95.9-100) |
|
Anti-Polio type 3 (≥1:8) |
88.2 (81.6-93.1) |
90.4 (84.2-94.8) |
100 (97.5-100) |
98.6 (92.7-100) |
100 (95.4-100) |
|
Anti-PRP (≥0.15
µg/mL)d |
100 (97.3-100) |
100 (97.3-100) |
100 (97.7-100) |
99.0 (94.8-100) |
99.1 (94.9-100) |
|
Anti-PRP (≥1 µg/mL)d |
92.7 (86.9-96.4) |
89.0 (82.5-93.7) |
93.6 (88.5-96.9) |
NR |
NR |
|
Vaccine response (for pertussis) |
|
Anti-PTe,f,g |
68.4 (59.9-76.1)e |
66.2 (57.6-74.1) |
93.8 (88.6-97.1)f |
100 (96.5-100)g |
99.0 (94.8-100) |
|
Anti-FHAf.g |
NR |
NR |
99.3 (96.3-100)f |
97.0 (91.6-99.4)g |
98.0 (93.1-99.8) |
|
Anti-PRNg |
NR |
NR |
NR |
99.0 (94.8-100)g |
99.0 (94.8-100) |
|
Pertussis IgGe |
75.7 (67.6-82.7)e |
72.8 (64.5-80.1) |
NR |
NR |
NR |
|
Data are % (95% CI). *Pentavac SD (Serum
Institute of India Ltd) and Imovax Polio (Sanofi
Pasteur India Pvt. Ltd); aIt is not clear
whether all infants in the wP-hexa study
[37] received a birth dose of HBV vaccine;
bWHO-defined levels for seroprotection
against diphtheria are 0.01 IU/mL (some
protection) and 0.1 IU/mL (full protection)
using a toxin neutralization test [41]. The
wP-hexa and 3aP-hexa studies used ELISA [37,
39]; the 2aP-hexa study used a
neutralization assay [38]; cWHO-defined
levels for seroprotection against tetanus
are 0.01 IU/mL (neutralization test or
modified ELISA) and 0.1-0.2 IU/mL (standard
ELISA) [67]. The wP-hexa study used a
specific ELISA [38]; the 2aP-hexa study
ELISA [39]; the 3aP-hexa study standard
ELISA [38]; dWHO-defined levels for
seroprotection against Hib are 0.15 µg/mL
(short-term protection) and 1 µg/mL
(long-term protection) [68]; eIf
seronegative pre-vaccination: ³100 µg/mL for
anti-PT or ³18 IU/mL for pertussis IgG; if
seropositive pre-vaccination: ³4-fold
increase in antibody titer level; fIf
pre-vaccination concentrations <4 × LLOQ: ³4
× LLOQ of the assay (2 IU/mL); if
pre-vaccination concentrations ³4 × LLOQ,
³pre-vaccination concentration; gIf
seronegative pre-vaccination: ³5 EL.U/mL; if
seropositive pre-vaccination: ³1-fold
increase in antibody concentration; CI:
confidence interval; ELISA: enzyme-linked
immunosorbent assay; FHA: filamentous
hemagglutinin; HBs: hepatitis B surface
antigen; HBV: hepatitis B virus; Hib:
Haemophilus influenzae type b; IgG:
immunoglobulin G; LLOQ: lower limit of
quantification; NR: not reported; PRN:
pertactin; PRP: polyribosylribitol
phosphate; PT: pertussis toxoid. |
Vaccine response (pertussis
antigens) and seroprotection (other antigens)
results 1 month after primary vaccination with a
hexavalent vaccine at 6-10-14 weeks are shown in
Table II. It should be noted that the
definitions of vaccine response and seroprotection
varied between the studies [37-40]. As there is no
established correlate of protection for pertussis,
anti-PT or pertussis immunoglobulin G levels were
used to assess vaccine response against pertussis
components and considered as surrogate markers for
protection. Pertussis vaccine response results for
wP-hexa were comparable to wP-penta for anti-PT
(68.4% vs. 66.2%) and pertussis
immunoglobulin G (75.7% vs. 72.8%);
seroprotection rates for the other antigens were
88.2-100% [37] (Table II). For
2aP-hexa, pertussis vaccine response results were
93.8% (anti-PT) and 99.3% (anti-FHA) [38].
Seroprotection rates were >99% for most antigens.
Diphtheria seroprotection rates were 99.3% and
49.6%, respectively based on anti-diphtheria
antibodies cut-off of 0.01 IU/mL and the
WHO-recommended full protective cut-off (0.1 IU/mL)
[38,41]. For 3aP-hexa, vaccine response rates for
the three pertussis antigens were 97.0-100% and
seroprotection rates for the other five antigens
were 98.6-100% [39].
TABLE III Adverse Events After Vaccination with Hexavalent Vaccine at 6-10-14 weeks in Indian Infantsa
|
wP-hexa [37] |
2aP-hexa [38] |
3aP-hexa [39, 40] |
|
wP-hexa |
Control arm |
2aP-hexa (n=177) |
3aP-hexa(n=111) |
Control arm
|
|
(n=142) |
(n=142) wP-penta
|
Post-dose 3 |
61014 wk group |
(n=112)3aP- |
|
3 doses |
+ Polio*3 doses |
|
|
hexa 246 mo |
|
|
|
|
|
group |
|
Solicited local AEs |
|
Pain/tenderness |
50.7 |
52.1 |
30.5 (23.7-37.9) |
25.2 (17.5-34.4) |
13.4 (7.7-21.1) |
|
Grade 3 |
NR |
NR |
NR |
1.8 (0.2-6.4) |
0.9 (0.0-4.9) |
|
Swelling |
24.6 |
15.5 |
14.9 (10.0-21.1) |
7.2 (3.2-13.7) |
8.0 (3.7-14.7) |
|
Grade 3 |
NR |
NR |
NR |
0.9 (0-4.9) |
0.9 (0-4.9) |
|
Redness/erythema |
19.0 |
9.2 |
7.5 (4.0-12.4) |
5.4 (2.0-11.4) |
1.8 (0.2-6.3) |
|
Grade 3 |
NR |
NR |
NR |
0 (0-3.3) |
0 (0-3.2) |
|
Solicited systemic AEs |
|
Fever/temperature |
57.0 |
52.1 |
19.0 (13.4-25.6) |
15.3 (9.2-23.4) |
15.2 (9.1-23.2) |
|
Grade 3 |
NR |
NR |
0 (0-0.2) |
0 (0-3.3) |
0.9 (0.0-4.9) |
|
Irritability/restlessness/fussiness |
7.7 |
7.7 |
36.2 (29.1-43.8) |
11.7 (6.4-19.2) |
8.9 (4.4-15.8) |
|
Grade 3 |
NR |
NR |
0.6 (0-3.2) |
0 (0-3.3) |
0 (0-3.2) |
|
Vomiting |
1.4 |
0.7 |
14.9 (10.0-21.1) |
NR |
NR |
|
Grade 3 |
NR |
NR |
0 (0-0.2) |
NR |
NR |
|
Sleepiness/drowsiness |
0.7 |
1.4 |
13.2 (8.6-19.2) |
0 (0-3.3) |
1.8 (0.2-6.3) |
|
Grade 3 |
NR |
NR |
1.1 (0.1-4.1) |
0 (0-3.3) |
0 (0-3.2) |
|
Loss of appetite |
0 |
1.4 |
10.9 (6.7-16.5) |
1.8 (0.2-6.4) |
4.5 (1.5-10.1) |
|
Grade 3 |
NR |
NR |
0 (0-0.2) |
0 (0-3.3) |
0 (0-3.2) |
|
Acute allergic reaction |
0.7 |
0 |
NR |
NR |
NR |
|
Grade 3 |
NR |
NR |
NR |
NR |
NR |
|
Unsolicited AEs |
0.7 |
1.4 |
20.3 |
35.7 |
22.3 |
|
Grade 3 |
NR |
NR |
NR |
0 |
0 |
|
SAE |
0.7 |
0 |
1.7 |
1.8 |
2.7 |
|
Data are % any grade (% grade 3); *Pentavac
SD (Serum Institute of India Ltd) and Imovax
Polio (Sanofi Pasteur India Pvt. Ltd);
aDuring 4 [37], 7 [38], or 4 days [39, 40];
AE: adverse event; CI, confidence interval;
m: months; NR: not reported; SAE: serious
adverse event. |
In the study that compared
wP-hexa with pentavalent DTwP-HBV-Hib plus IPV,
immunogenicity results were comparable with both
regimens [37]. Similarly, in the study that compared
two different dosing schedules (6-10-14 weeks and
2-4-6 months) of 3aP-hexa, immunogenicity results
were similar with both schedules [39].
Safety results for the three
Indian studies are summarized in Table III
[37-40]. The most common solicited local adverse
events (AEs) were pain/tenderness (wP-hexa and
2aP-hexa) and pain (3aP-hexa); while the most common
solicited systemic AEs were fever (wP-hexa),
irritability (2aP-hexa), and temperature (3aP-hexa)
[37-40]. Serious adverse events were rare (<2% in
each study) and none were judged to be related to
vaccination [37-39]. All three studies reported that
the hexavalent vaccines were well tolerated [37-39].
In the study that compared
wP-hexa with pentavalent DTwP-HBV-Hib plus IPV,
reactogenicity and safety results were comparable
with both regimens [37]. In the study that compared
two different dosing schedules of 3aP-hexa, safety
results were similar, although pain was more often
reported in the 6-10-14-week group vs. the
2-4-6-month group (25.2% vs 13.4%) [39].
Booster Dose
Published studies that have
tested the immune response to a booster dose of
hexavalent vaccine in Indian infants are not
available but two studies one for 2aP-hexa in
South Africa [32] and one for 3aP-hexa in the
Philippines [31] have reported on the immune
response after the booster dose following a
6-10-14-week primary schedule. In the first part of
the South African study, infants were randomized to
2aP-hexa (with [n=143] or without [n=286]
birth HBV) or DTwP-Hib plus HBV plus OPV vaccines (n=286)
at 6-10-14 weeks [42]. Among infants who received
2aP-hexa, those who received the birth HBV vaccine
dose were more likely to obtain anti-HBs
³10
mIU/mL (99.0% vs 95.7%) [42]. In the second part of
the study, infants received the same vaccine(s) as
boosters at 15-18 months of age [32]. Seroprotection
rates one month after the booster were 100% for all
antigens apart from the pertussis antigens, for
which vaccine response rates were 93.9% (anti-PT)
and 94.7% (anti-FHA) [32] (Table IV).
TABLE IV Seroprotection/Vaccine Response Rates 1 Month After Booster Vaccination in Children Who Had Received
A Birth Dose of HBV Vaccine and Three Doses (at 6-10-14 weeks) of Hexavalent Vaccine
|
Vaccine, Country |
2aP-hexa [32], South Africa |
3aP-hexa [31], Philippines |
|
|
15-18 |
|
|
12-15 |
|
|
Age at booster dose (mo) |
Group 1 (n=218)primary series ofDTaP-IPV-HepB-PRP-T,
with noHBV at birth |
Group 2 (n=219)primary series ofDTwP-Hib+hepatitisB+OPV,
with noHBV at birth |
Group 3 (n=130)primary series of
DTaP-IPV-Hep B-PRP-T, with
HBV at birth |
No HBV at birth(n=111) |
HBV at birth (n=111) |
|
Seroprotectiona |
|
Anti-diphtheria (≥0.1 IU/mL) |
100 (98.1-100) |
99.0 (96.4-99.9) |
100 (96.7-100) |
99.0 (94.6-100) |
100 (96.7-100) |
|
Anti-tetanus (≥0.1 IU/mL) |
100 (98.2-100) |
100 (98.2-100) |
100 (96.8-100) |
99.0 (94.6-100) |
99.1 (95.0-100) |
|
Anti-HBs (≥10 mIU/mL) |
98.5 (95.6-99.7) |
NA |
100 (96.8-100) |
90.0 (82.4-95.1) |
99.1 (95.0-100) |
|
Anti-Polio type 1 (≥1:8) |
100 (98.1-100) |
97.4 (94.0-99.1) |
100 (96.6-100) |
100 (95.8-100) |
100 (95.9-100) |
|
Anti-Polio type 2 (≥1:8) |
100 (98.1-100) |
100 (98.1-100) |
100 (96.6-100) |
100 (95.7-100) |
100 (95.8-100) |
|
Anti-Polio type 3 (≥1:8) |
100 (98.1-100) |
98.9 (96.2-99.9) |
100 (96.6-100) |
100 (95.6-100) |
100 (95.8-100) |
|
Anti-PRP (≥0.15 µg/mL) |
100 (98.2-100) |
100 (98.2-100) |
100 (96.8-100) |
100 (96.4-100) |
100 (96.7-100) |
|
Anti-PRP (≥1 µg/mL) |
98.5 (95.7-99.7) |
98.5 (95.7-99.7) |
100 (96.8-100) |
99.0 (94.6-100) |
99.1 (95.1-100) |
|
Vaccine response (for pertussis) |
|
Anti-PTb,c |
94.8 (90.0-97.7) |
83.5 (76.0-89.3) |
93.9 (87.3-97.7)b |
99.0 (92.7-99.7) |
100 (96.5-100)c |
|
Anti-FHAb,c |
91.2 (85.7-95.1) |
96.5 (92.0-98.9) |
94.7 (88.0-98.3)b |
97.9 (94.6-100) |
100 (95.0-100)c |
|
Anti-PRNb,c |
NR |
NR |
NR |
99.0 (94.4-100) |
99.1 (96.6-100)c |
|
Data are % (95% CI) unless otherwise
indicated; aPlease see footnotes to Table II
for details about seroprotection cut-offs.
The 2aP-hexa study used seroneutralization
for diphtheria, ELISA for tetanus [32]; the
3aP-hexa study used standard ELISA for both
[31]; b ³4-fold
increase vs pre-booster; cIf seronegative
pre-booster: appearance of antibodies; if
seropositive pre-booster:
³2-fold
increase in antibody concentrations or
titers; CI: confidence interval; ELISA:
enzyme-linked immunosorbent assay; FHA:
filamentous hemagglutinin; HBs: hepatitis B
surface antigen; HBV: hepatitis B vaccine;
PRN: pertactin; PRP: polyribosylribitol
phosphate; PT: pertussis toxoid.
|
In the Philippines study, 320
minfants were randomized to 3aP-hexa at 6-10-14
weeks with (n=160) or without (n=160)
birth HBV; they then received the hexavalent vaccine
at 12-15 months of age [31]. Infants who received
the birth HBV vaccine dose were significantly more
likely to obtain anti-HBs
³10
mIU/mL after the primary series (98.5% vs.
77.7%) and after the booster (99.1% vs.
90.0%) than those who did not. Among those who
received a birth dose of HBV, vaccine response
(pertussis antigens) and seroprotection (other
antigens) rates one month after the booster dose
were all >99% [31] (Table IV).
Pertussis Efficacy
For most of the antigens included
in hexavalent vaccines, generally accepted
seroprotective cut-offs are available, and these can
be used to imply vaccine efficacy. However, there is
no defined correlate of protection for pertussis, so
efficacy has to be assessed in clinical studies. No
studies have directly assessed the efficacy of any
of the three hexavalent vaccines against pertussis
due to ethical and feasibility considerations, which
is why the current vaccines are licensed by the
regulators based on immunological non-inferiority
vs. historical vaccines or current standard of
care. However, in a 3-dose primary series study
using a DTaP vaccine with a DTaP component similar
to 2aP-hexas in a highly endemic country (Senegal),
vaccine efficacy against WHO-defined typical
pertussis ( ³21
days of paroxysmal cough) was 74% in DTaP arm and
92% in DTwP arm [43]. Similarly, the efficacy of
3-dose primary immunization with DTaP (Infanrix;
GSK) with a DTaP component similar to 3aP-hexas has
been reported to be 88.7% against typical pertussis
(³21
days of spasmodic cough with confirmed Bordetella
pertussis) in a prospective household contact
study in Germany [44]; while in an Italian study,
86% efficacy was shown up to 60 months after
completion of a 3-dose primary series [45]. As the
pertussis immune response to 3aP-hexa is
equivalent to that of the DTaP vaccine (Infanrix;
GSK), the protective efficacy of the two vaccines is
expected to be equivalent [35, 36].
Long-term Immune Response
To our knowledge, there are no
long-term immune persistence data for wP-hexa or for
any of the three vaccines in Indian subjects. For
2aP-hexa, seroprotection rates at age 4.5 years
after 3-dose primary series and a booster dose
(6-10-14 weeks and 15-18 months or 2-4-6 months and
12-24 months) are: 97.0-100% (anti-diphtheria
³0.01
IU/mL), 57.2-75.3% (anti-diphtheria
³0.1
IU/mL), 100% (anti-tetanus
³0.01
IU/mL), 80.8-89.5% (anti-tetanus
³0.1
IU/mL), 73.3% or 92.3-96.1% (anti-HBs
³10
mIU/mL without or with birth HBV, respectively),
98.8-100% (anti-PRP
³0.15
µg/mL), 22.2-42.5% (anti-PT
³8
EU/mL), 85.6-93.8% (anti-FHA
³8
EU/mL), and 99.5-100% (anti-polio types 1, 2, 3 (³1:8)
[46, 47].
Longer immune persistence data
following three primary 3aP-hexa vaccinations and a
booster dose in the second year of life have been
published [35, 36,48]. Seroprotective antibody
levels (among children who had not received
additional diphtheria/tetanus booster doses)
persisted up to 7-9 years of age in 99.5% of
children for Hib ( ³0.15
µg/mL), 91.0-97.2% for the three types of
poliomyelitis, 66.7% for diphtheria (³0.1
IU/mL), 72.1-77.2% for HBV (³10
mIU/mL), and 64.7% for tetanus (³0.1
IU/mL) [35,36,48]. Seropositivity (³5
EL.U/mL) for FHA and PRN were high (98.1% and 87.0%,
respectively), but for PT, this was only 32.3%
[35,36,48]. The low circulating anti-PT antibodies
may be indicative of an absence of pertussis
infection, suggesting that the vaccination program
was effective in preventing pertussis [48]. It is
well described that neither natural infection, nor
wP or aP vaccines provide life-long protection [25].
Furthermore, studies with both 2aP-hexa and 3aP-hexa
indicate waning of pertussis immune response which
is consistent with previous reports which reinforce
the need for booster vaccination against pertussis
[14,18].
Among children in Thailand who
had received a birth dose of HBV and three primary
doses of 2aP-hexa or 3aP-hexa, 49.3% or 42.9%,
respectively, had seroprotective anti-HBs levels at
9-10 years of age [49]. Further, a strong anamnestic
response (an enhanced reaction to an antigen related
to one previously encountered) was seen post-HBV
challenge revaccination in 92.8% and 98.7%,
respectively [49]. For 3aP-hexa, immune persistence
up to age 14-15 years has been shown after receipt
of four doses during infancy (no birth HBV vaccine
dose) [50]. Among 268 adolescents, 53.7% and 93.3%
had anti-HBs ³10
mIU/mL before and 1 month after, respectively, a
challenge dose of HBV vaccine [50].
Coadministration with Other
Childhood Vaccines
If hexavalent combination
vaccines are used in the Indian schedule, they would
likely be co-administered with rotavirus and/or
pneumococcal conjugate vaccines (PCVs) at 6-10-14
weeks [6,7], and could also be
co-administered with measles-mumps-rubella
(MMR), varicella, measles-mumps-rubella-varicella
(MMRV), and/or hepatitis A vaccines in the second
year of life [7].
There are no published studies of
wP-hexa co-administered with other routine vaccines,
but the product leaflet states that it can be given
at the same time as PCVs and MMR and rotavirus
vaccines [33].
2aP-hexa has been evaluated in
co-administration studies outside India, and data
suggest no clinically relevant interference on
concomitant administration with PCV, MMR, rotavirus,
or meningococcal conjugate vaccines [46]. In a South
African study in which 15-18-month-old toddlers
received a booster dose of 2aP-hexa concomitant with
MMR and varicella vaccines, the response to the
varicella vaccine was slightly lower than would be
expected [32]. Due to a potentially clinically
relevant interference in the antibody response of
varicella vaccine, 2aP-hexa and varicella vaccines
should not be administered at the same time [46].
Various studies have examined the
effects on immunogenicity and safety of
co-administering 3aP-hexa vaccine with other
childhood vaccines outside India: PCVs [51,52] and
rotavirus [53], MMRV [54,55], and meningococcal
[56-61] vaccines. In all studies, the immune
responses remained robust when the vaccines were
co-administered, with no clinically relevant
interference in the antibody response to each of the
antigens [35,36]. Febrile reactions were more common
when 3aP-hexa vaccine was administered concomitantly
with a PCV or MMRV, but these are mostly moderate ( £39°C)
and transient [35,36]. A case-control study reported
higher local reactogenicity of 3aP-hexa vs.
DTaP-IPV-Hib vaccines when co-administered with MMRV
vaccine at 18 months of age [55]. For PCV
co-administration, fever has been reported to occur
less frequently with 3aP-hexa than 2aP-hexa when
co-administered with PCV in randomized controlled
trials. In one such trial, following a three-dose
primary schedule, fever rates of 72.8% (95% CI
67.0-78.1%) and 56.7% (95% confidence interval [CI]
50.5-62.8%) were reported in children who received a
PCV plus rotavirus vaccine plus either 2aP-hexa or
3aP-hexa, respectively [62]. A similar trend was
seen following the second-year booster vaccination
(given with a PCV only), with fever seen in 50.2%
(95% CI 43.6-56.8%) and 43.6% (95% CI 37.1-50.3%),
respectively [62].
Preterm Infants
3aP-hexa is the only hexavalent
vaccine available in India that has prospective
clinical data in preterm infants (and includes such
information in its label) which indicates that
3aP-hexa has a similar immunogenicity and safety
profile in preterm and full-term infants.
Cardiorespiratory events in preterm infants of <28
weeks gestation were observed, but this seemed to be
influenced by the infantss underlying condition as
the cardiorespiratory risk in this population is a
point of attention for the prescriber/vaccinator in
general and most resolved spontaneously or with
minimal intervention [63].
Conclusions
Use of combination vaccines is a
practical way to reduce the number of injections
given to infants and children. Vaccination schedules
can also be simplified with the use of hexavalent
combination vaccines for primary and booster
vaccination. Three IPV-containing hexavalent
vaccines are available in India. The level of
available evidence and experience with these three
vaccines vary widely [64-66]. All three vaccines
evoke immune responses to their contained antigens
in phase 3 studies in Indian children using the
6-10-14-week schedule for the primary series
[37-39]. 2aP-hexa and 3aP-hexa have also been shown
to be immunogenic when tested as a booster dose in
the second year of life following a 6-10-14-week
primary series [31,32]. All three hexavalent
vaccines are well tolerated; although whole-cell
pertussis containing vaccines may result in more
solicited local reactions and fever than those with
acellular pertussis components.
Acknowledgements: The
authors would like to thank the Business & Decision
Life Sciences platform for editorial assistance and
manuscript coordination, on behalf of GSK. Thibaud
André (Business & Decision Life Sciences)
coordinated the manuscript development and provided
editorial support. Jenny Lloyd (Compass Medical
Communications Ltd.) provided writing support.
Contributors: All
authors provided substantial intellectual and
scientific input during manuscript development,
critically reviewed the content, revised the
manuscript, and approved the final version.
Funding:
GlaxoSmithKline Biologicals SA took charge of all
costs associated with the development and
publication of this manuscript.
Competing interests: AC: has
received lecture fees and advisory board fees from
Sanofi Pasteur and Abbott Vaccines; RP,AM,SK: are
employees of the GSK group of companies; AM: has
received shares from the GSK group of companies.
Trademarks: Hexaxim is
a trademark of Sanofi Pasteur; Imovax Polio
is a trademark of Sanofi Pasteur India Pvt. Ltd.;
Pentavac SD is a trademark of Serum Institute of
India Ltd.; EasySix is a trademark of Panacea
Biotec Ltd.; Infanrix hexa and Infanrix
are trademarks of the GSK group of companies.
References
1. Skibinski DA, Baudner BC,
Singh M, OHagan DT. Combination vaccines. J Glob
Infect Dis. 2011;3:63-72.
2. Advisory Committee on
Immunization Practices (ACIP). Combination vaccines
for childhood immunization. MMWR Recomm Rep.
1999;48:1-14.
3. Maman K, Zollner Y, Greco D,
Duru G, Sendyona S, Remy V. The value of childhood
combination vaccines: From beliefs to evidence. Hum
Vaccin Immunother. 2015;11:2132-41.
4. Obando-Pacheco P, Rivero-Calle
I, Gomez-Rial J, Rodriguez-Tenreiro Sanchez C,
Martinon-Torres F. New perspectives for hexavalent
vaccines. Vaccine. 2018;36:5485-94.
5. Orsi A, Azzari C, Bozzola E,
Chiamenti G, Chirico G, Esposito S, et al.
Hexavalent vaccines: characteristics of available
products and practical considerations from a panel
of Italian experts. J Prev Med Hyg.
2018;59:E107-E19.
6. National Health Mission.
Current UIP Schedule. [Available from:
http://www.nhm.gov.in/nrhm-components/rmnch-a/immunization/manual-formats.html.
Accessed January 31, 2019.
7. Balasubramanian S, Shah A,
Pemde HK, Chatterjee P, Shivananda S, Guduru VK,
et al. Indian Academy of Pediatrics (IAP)
Advisory Committee on Vaccines and Immunization
Practices (ACVIP) Recommended Immunization Schedule
(2018-19) and Update on Immunization for Children
Aged 0 Through 18 Years. Indian Pediatr.
2018;55:1066-74.
8. World Health Organization.
Replacing Trivalent OPV with Bivalent OPV. 2015.
Available from:
https://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2
/oral_polio_vaccine/en/. Accessed May 29, 2019.
9. Haldar P, Agrawal P. Indias
preparedness for introduction of IPV and switch from
tOPV to bOPV. Indian Pediatr. 2016;53:S44-S9.
10. Kumar A, Basu S, Vashishtha
V, Choudhury P. Burden of rotavirus diarrhea in
under five indian children. Indian Pediatr.
2016;53:607-17.
11. World Health Organization.
Introduction of Inactivated Polio Vaccine (IPV) in
Routine Immunizations. Available from:
https://www.who.int/immunization/diseases/polio
myelitis/inactivated_polio_vaccine/ipv_operational_
manual.pdf. Accessed January 30, 2019.
12. World Health Organization.
Update on short term supply constraints for IPV.
2015. Available from:
https://www.who.int/immunization/diseases/
poliomyelitis/endgame_objective2/inactivated_polio_vaccine/IPVSupplyInformationNote-June2015_FINAL.pdf.
Accessed January 30, 2019.
13. Bahl S, Bhatnagar P, Sutter
RW, Roesel S, Zaffran M. Global polio eradication -
way ahead. Indian J Pediatr. 2018;85:124-31.
14. World Health Organization.
Pertussis vaccines: WHO position paper - September
2015. Releve epidemiologique hebdomadaire.
2015;90:433-58.
15. Jefferson T, Rudin M,
DiPietrantonj C. Systematic review of the effects of
pertussis vaccines in children. Vaccine.
2003;21:2003-14.
16. World Health Organization.
Observed Rate of Vaccine Reactions: Diphtheria,
Pertussis, Tetanus Vaccines. 2014. Available from:
https://www.who.int/vaccine_safety/initiative/tools/DTP_vaccine_rates_information_
sheet.pdf?ua=1. Accessed January 30, 2019.
17. Zhang L, Prietsch SO,
Axelsson I, Halperin SA. Acellular vaccines for
preventing whooping cough in children. Cochrane
Database Syst Rev. 2014:CD001478.
18. World Health Organization.
Pertussis vaccines: WHO position paper - August
2015. Releve Epidemiologique Hebdomadaire.
2015;90:433-60.
19. Dowling DJ. Recent advances
in the discovery and delivery of TLR7/8 agonists as
vaccine adjuvants. Immuno Horizons. 2018;2:185-97.
20. Reed SG, Orr MT, Fox CB. Key
roles of adjuvants in modern vaccines. Nature
Medicine. 2013;19:1597.
21. Misiak A, Leuzzi R, Allen AC,
Galletti B, Baudner BC, DOro U, et al.
Addition of a TLR7 agonist to an acellular pertussis
vaccine enhances Th1 and Th17 responses and
protective immunity in a mouse model. Vaccine.
2017;35:5256-63.
22. Cellčs MDd, Magpantay FMG,
King AA, Rohani P. The pertussis enigma: reconciling
epidemiology, immunology and evolution. Proc Biol
Sci. 2016;283:20152309.
23. Fernandes EG, Sartori AMC, de
Soįrez PC, Carvalhanas TRMP, Rodrigues M, Novaes
HMDJBID. Challenges of interpreting epidemiologic
surveillance pertussis data with changing diagnostic
and immunization practices: The case of the state of
Sćo Paulo, Brazil. BMC Infect Dis.
2018;18:126.
24. WHO SAGE pertussis working
group. Background paper. Available from:
https://www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_
FINAL4_ web.pdf?ua=. Accessed February 15, 2019.
25. Diavatopoulos DA, Mills KHG,
Kester KE, Kampmann B, Silerova M, Heininger U,
et al. PERISCOPE: road towards effective control
of pertussis. Lancet Infect Dis. 2019;19:e179-e86.
26. Chitkara AJ, Vashistha VM.
Pertussis outbreaks in the developed world: Are
acellular pertussis vaccines ineffective? Indian
Pediatr. 2013;50:1109-12.
27. Domenech de Celles M,
Magpantay FM, King AA, Rohani P. The pertussis
enigma: reconciling epidemiology, immunology and
evolution. Proc Biol Sci. 2016;283(1822).
28. Vashishtha VM, Bansal CP,
Gupta SG. Pertussis vaccines: Position paper of
Indian Academy of Pediatrics (IAP). Indian Pediatr.
2013;50:1001-9.
29. Patterson J, Kagina BM, Gold
M, Hussey GD, Muloiwa R. Comparison of adverse
events following immunisation with acellular and
whole-cell pertussis vaccines: A systematic review.
Vaccine. 2018;36:6007-16.
30. Dolhain J, Fierens F, De
Moerlooze L, Nissen M, Janssens W, Mukherjee P.
Integration of hepatitis B vaccine (HBV) and DTPa-IPV/Hib
immunization schedules: overview of clinical
experience with GSK HBV vaccine, DTPa-IPV/Hib and
DTPa-HBV-IPV/Hib in the Asian region. 2017.
Available from:
https://wspid2017.kenes.com/Documents/WSPID17_-all%20abstracts.pdf.
Accessed August 7, 2018.
31. Gatchalian S, Bravo L,
Cadrona-Carlos J, Espos R, Fortunato T, Hernandez-Tanueco
V, et al. A hexavalent DTPa-HBV-IPV/Hib
vaccine administered to Filipino infants at 6, 10
and 14 weeks and 12-15 months of age; importance of
the birth dose of HBV. Philipp J Pediatr.
2007;56:153-61.
32. Madhi SA, Koen A, Cutland C,
Groome M, Santos-Lima E. Antibody persistence and
booster vaccination of a fully liquid hexavalent
vaccine coadministered with measles/mumps/rubella
and varicella vaccines at 15-18 months of age in
healthy South African infants. Pediatr Infect Dis J.
2013;32:889-97.
33. Panacea Biotec. Purified
Diphtheria Toxoid, Purified Tetanus Toxoid, Whole
cell Pertussis, Recombinant Hepatitis B,
Haemophilus influenzae Type b Conjugate and
Inactivated Poliomyelitis Trivalent Vaccine
(Adsorbed) [EasySix]. Available from: https://media.bestonhealth.
in/documents/2018/8/11/EasysixPIPMPIS05903.pdf.
Accessed February 18, 2019.
34. Sanofi Pasteur. Hexaxim
Summary of Product Characteristics. Available from:
http://www.ema.europa. eu/docs/en_GB/document_library/Medicine_for_use_
outside_EU/2012/12/WC500135727.pdf. Accessed
June 11, 2018.
35. GlaxoSmithKline UK. Infanrix
hexa Summary of Product Characteristics. Availalble
from: http://www.ema. europa.eu/ema/index.jsp?curl=pages/
medicines/human/medicines/000296/human_med_000833.jsp&mid=
WC0b01ac058001d124. Accessed June 11, 2018.
36. GSK. Prescribing Information
(Infanrix hexa). Available from:http://india-pharma.gsk.com/en-in/products/prescribing-information/.
Accessed: August 14, 2018.
37. Mohanty L, Sharma S, Behera
B, Panwar S, Paliwal C, Gupta A, et al. A
randomized, open label trial to evaluate and compare
the immunogenicity and safety of a novel liquid
hexavalent DTwP-Hib/Hep B-IPV (EasySix) to licensed
combination vaccines in healthy infants. Vaccine.
2018;36:2378-84.
38. Chhatwal J, Lalwani S, Vidor
E. Immunogenicity and safety of a liquid hexavalent
vaccine in Indian infants. Indian Pediatr.
2017;54:15-20.
39. Lalwani SK, Agarkhedkar S,
Sundaram B, Mahantashetti NS, Malshe N, Agarkhedkar
S, et al. Immunogenicity and safety of 3-dose
primary vaccination with combined DTPa-HBV-IPV/Hib
in Indian infants. Hum Vaccin Immunother.
2017;13:120-7.
40. GSK. Immunogenicity and
safety study of GlaxoSmithKline Biologicals
Infanrix hexa vaccine in healthy infants in India.
Infanrix hexaTM (DTPa-HBV-IPV/Hib): GlaxoSmithKline
(GSK) Biologicals combined diphtheria-tetanus-acellular
pertussis-hepatitis B-inactivated poliovirus and
Haemophilus influenzae (H. influenzae)
Type b vaccine. 2016. Available from:
https://www.gsk-clinicalstudyregister.com/files2/111157%20-%20Clinical-Study-Result-Summary.pdf.
Accessed June 26, 2018.
41. World Health Organization.
India: WHO and UNICEF estimates of national
immunization coverage: 2016 revision. 2017.
Available from:
http://www.who.int/immunization/monitoring_surveillance/data/ind.pdf.
Accessed September 11, 2017.
42. Madhi SA, Mitha I, Cutland C,
Groome M, Santos-Lima E. Immunogenicity and safety
of an investigational fully liquid hexavalent
combination vaccine versus licensed combination
vaccines at 6, 10, and 14 weeks of age in healthy
South African infants. Pediatr Infect Dis J.
2011;30:e68-74.
43. Simondon F, Preziosi MP, Yam
A, Kane CT, Chabirand L, Iteman I, et al. A
randomized double-blind trial comparing a
two-component acellular to a whole-cell pertussis
vaccine in Senegal. Vaccine. 1997;15:1606-12.
44. Schmitt HJ, von Konig CH,
Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H,
et al. Efficacy of acellular pertussis vaccine in
early childhood after household exposure. JAMA.
1996;275:37-41.
45. Salmaso S, Mastrantonio P,
Tozzi AE, Stefanelli P, Anemona A, Ciofi degli Atti
ML, et al. Sustained efficacy during the first 6
years of life of 3-component acellular pertussis
vaccines administered in infancy: the Italian
experience. Pediatrics. 2001;108:E81.
46. Hexaxim. Summary of Product
Characteristics. Sanofi Pasteur SA. 2012;Available
from: http://www.ema. europa.eu/ema/index.jsp?curl=pages/medicines/
document_ listing/document_listing_ 000352. jsp&mid=.
Accessed Jan 2018.
47. Madhi SA, Lopez P, Zambrano
B, Jordanov E, BChir S, Noriega F, et al.
Antibody persistence in pre-school children after
hexavalent vaccine infant primary and booster
administration. Hum Vaccin Immunother. 2018: 1-11.
48. Zinke M, Disselhoff J,
Gartner B, Jacquet JM. Immunological persistence in
4-6 and 7-9 year olds previously vaccinated in
infancy with hexavalent DTPa-HBV-IPV/Hib. Human
vaccines. 2010;6:189-93.
49. Kosalaraksa P,
Chokephaibulkit K, Benjaponpitak S, Pancharoen C,
Chuenkitmongkol S, BChir S, et al.
Persistence of hepatitis B immune memory until 9-10
years of age following hepatitis B vaccination at
birth and DTaP-IPV-HB-PRP approximately T
vaccination at 2, 4 and 6 months. Hum Vaccin
Immunother. 2018;14:1257-65.
50. Schwarz TF, Behre U, Adelt T,
Donner M, Suryakiran PV, Janssens W, et al.
Long-term antibody persistence against hepatitis B
in adolescents 14-15-years of age vaccinated with 4
doses of hexavalent DTPa-HBV-IPV/Hib vaccine in
infancy. Hum Vaccin Immunother. 2019;15:235-41.
51. Knuf M, Habermehl P, Cimino
C, Petersen G, Schmitt HJ. Immunogenicity,
reactogenicity and safety of a 7-valent pneumococcal
conjugate vaccine (PCV7) concurrently administered
with a DTPa-HBV-IPV/Hib combination vaccine in
healthy infants. Vaccine. 2006;24:4727-36.
52. Esposito S, Tansey S,
Thompson A, Razmpour A, Liang J, Jones TR, et al.
Safety and immunogenicity of a 13-valent
pneumococcal conjugate vaccine compared to those of
a 7-valent pneumococcal conjugate vaccine given as a
three-dose series with routine vaccines in healthy
infants and toddlers. Clin Vaccine Immunol.
2010;17:1017-26.
53. Vesikari T, Karvonen A,
Prymula R, Schuster V, Tejedor JC, Thollot F, et al.
Immunogenicity and safety of the human rotavirus
vaccine Rotarix co-administered with routine infant
vaccines following the vaccination schedules in
Europe. Vaccine. 2010;28:5272-9.
54. Zepp F, Behre U, Kindler K,
Laakmann KH, Pankow-Culot H, Mannhardt-Laakmann W,
et al. Immunogenicity and safety of a tetravalent
measles-mumps-rubella-varicella vaccine
co-administered with a booster dose of a combined
diphtheria-tetanus-acellular pertussis-hepatitis
B-inactivated poliovirus-Haemophilus influenzae
type b conjugate vaccine in healthy children aged
12-23 months. Eur J Pediatr. 2007;166:857-64.
55. Kiely M, Billard MN, Toth E,
Zafack JG, Landry M, Skowronski DM, et al.
Investigation of an increase in large local
reactions following vaccine schedule change to
include DTaP-HB-IPV-Hib (Infanrix-hexa(R)) and MMRV
(ProQuad(R)) at 18months of age. Vaccine.
2018;36:6688-94.
56. Tejedor JC, Omenaca F,
Garcia-Sicilia J, Verdaguer J, Van Esso D, Esporrin
C, et al. Immunogenicity and reactogenicity
of a three-dose primary vaccination course with a
combined diphtheria-tetanus-acellular pertussis-hepatitis
B-inactivated polio-Haemophilus influenzae
type b vaccine coadministered with a meningococcal C
conjugate vaccine. Pediatr Infect Dis J.
2004;23:1109-15.
57. Tejedor JC, Moro M,
Ruiz-Contreras J, Castro J, Gomez-Campdera JA,
Navarro ML, et al. Immunogenicity and reactogenicity
of primary immunization with a hexavalent
diphtheria-tetanus-acellular pertussis-hepatitis
B-inactivated polio-Haemophilus influenzae
type B vaccine coadministered with two doses of a
meningococcal C-tetanus toxoid conjugate vaccine.
Pediatr Infect Dis J. 2006;25:713-20.
58. Knuf M,
Pantazi-Chatzikonstantinou A, Pfletschinger U,
Tichmann-Schumann I, Maurer H, Maurer L, et al.
An investigational tetravalent meningococcal
serogroups A, C, W-135 and Y-tetanus toxoid
conjugate vaccine co-administered with Infanrix hexa
is immunogenic, with an acceptable safety profile in
12-23-month-old children. Vaccine. 2011;29:4264-73.
59. Vesikari T, Esposito S,
Prymula R, Ypma E, Kohl I, Toneatto D, et al.
Immunogenicity and safety of an investigational
multicomponent, recombinant, meningo-coccal
serogroup B vaccine (4CMenB) administered
concomitantly with routine infant and child
vaccinations: results of two randomised trials.
Lancet. 2013;381:825-35.
60. Prymula R, Esposito S,
Zuccotti GV, Xie F, Toneatto D, Kohl I, et al.
A phase 2 randomized controlled trial of a
multicomponent meningococcal serogroup B vaccine
(I). Hum Vaccin Immunother. 2014;10:1993-2004.
61. Gossger N, Snape MD, Yu LM,
Finn A, Bona G, Esposito S, et al.
Immunogenicity and tolerability of recombinant
serogroup B meningococcal vaccine administered with
or without routine infant vaccinations according to
different immunization schedules: a randomized
controlled trial. JAMA. 2012;307:573-82.
62. Prymula R, Kieninger D,
Feroldi E, Jordanov E, BChir S, DaCosta X.
Immunogenicity and Safety of Primary and Booster
Vaccinations of a Fully Liquid DTaP-IPV-HB-PRP-T
Hexavalent Vaccine in Healthy Infants and Toddlers
in Germany and the Czech Republic. Pediatr Infect
Dis J. 2018:[Epub ahead of print].
63. Omenaca F, Vazquez L, Garcia-Corbeira
P, Mesaros N, Hanssens L, Dolhain J, et al.
Immunization of preterm infants with GSKs
hexavalent combined diphtheria-tetanus-acellular
pertussis-hepatitis B-inactivated poliovirus-Haemophilus
influenzae type b conjugate vaccine: A review of
safety and immunogenicity. Vaccine. 2018;36:986-96.
64. Public Health England. The
Hexavalent DTaP/IPV/Hib/HepB Combination Vaccine.
Available from:
https://assets.publishing.service.gov.uk/government/
uploads/system/uploads/attachment_data/file/740422/Infanrix_hexa_training_slides.pdf.
Accessed March 20, 2019.
65. ClinicalTrials.gov search.
2019. Available from:
https://clinicaltrials.gov/ct2/results?term=Infanrix+hexa&lead=
GlaxoSmithKline. Accessed May 31, 2019.
66. EU Clinical Trials Register.
2019. Available from:
https://www.clinicaltrialsregister.eu/ctr-search/search?query
=Infanrix+hexa +AND+GlaxoSmithKline+Biologicals.
Accessed May 31, 2019.
67. World Health Organization.
Tetanus vaccines: WHO position paper - February
2017. Releve Epidemiologique Hebdomadaire.
2017;92:53-76.
68. World Health Organization. Haemophilus
influenzae type b (Hib) Vaccination Position
Paper - September 2013. Releve Epidemiologique
Hebdomadaire. 2013;88:413-28.
|
|
|
 |
|

