|
|
|
Indian Pediatr 2019;56: 933-937 |
 |
Development, Cognition, Adaptive Function and
Maladaptive Behavior in HIV-infected and HIV-exposed Uninfected
Children Aged 2-9 Years
|
|
Sharmila Banerjee Mukherjee 1,
Shilpa Devamare1,
Anju Seth1 and
Savita Sapra2
From Departments of Pediatrics, 1Lady
Hardinge Medical College and associated hospitals; and 2All
India Institute of Medical Sciences; New Delhi, India.
Correspondence to: Dr Sharmila B Mukherjee,
Professor, Department of Pediatrics, Lady Hardinge Medical College and
associated hospitals, New Delhi, India.
Email: [email protected]
Received: March 24, 2019;
Initial review: April 29, 2019;
Accepted: August 03, 2019.
|
|
Objectives: To compare
development/cognition, adaptive function and maladaptive behavior of
HIV-infected and HIV-exposed uninfected children between 2 to 9 years
with HIV-uninfected controls. Methods: This hospital-based
cross-sectional study was conducted from November, 2013 to March, 2015.
50 seropositive HIV-infected, 25 HIV-exposed uninfected and 25
HIV-uninfected children between 2 to 9 years were administered
Developmental Profile 3, Vineland Adaptive Behavior Scale 2, and Child
Behavior Checklist for assessing development, adaptive function and
maladaptive behaviour, respectively. Additional data were obtained by
history, examination and review of records. Results:
Significant developmental/cognitive impairment was observed in 38 (76%),
16 (64%) and 6 (24%) HIV-infected, HIV-exposed uninfected, and
HIV-uninfected children, respectively. Significant impairment in
adaptive function was found in 12 (24%) and 2 (8%) HIV-infected and
HIV-exposed uninfected children, respectively. Maladaptive behavior was
not seen in any group. Conclusions: High magnitude of
impaired development/cognition and adaptive function in HIV-exposed and
HIV-infected children warrants assessment of these domains during
follow-up of these children, and incorporation of interventions for
these deficits in standard care for this group.
Keywords: Acquired immunodeficiency disorder,
Developmental delay, Neurocognition, Outcome.
|
|
T
he eco-biodevelopmental framework of child
development conceptualizes that interactions between ecology (social and
physical rearing environments) and biologic processes (heath), determine
developmental trajectories of early childhood. These influence
cognition, adaptive function (skills used in activities of daily living)
and behavior, the effects of which persist in late childhood,
adolescence and even adulthood [1]. Human Immunodeficiency virus (HIV)-
infected children from low- and middle-income countries (LMICs) are
exposed to more adverse factors than high- income countries. These
include non-detection or late detection in pregnancy and infancy,
unavailability of timely anti-retroviral therapy (ART), adverse
environ-mental (malnutrition, micro-deficiencies, decreased
opportunities, poor nurturing care, etc.) and social (i.e.
poverty, parental morbidity, mortality and poor literacy) factors [2,3].
HIV-exposed uninfected children are a less recognized, but equally
vulnerable population who lack HIV-related biological risk factors, but
otherwise face similar adversities.
Previous studies studying development, adaptive
function and behavior of these children from high-income countries
displayed differences varying from no impairment [4] to significant
impairment [5,6]. These discrepancies were explained by heterogeneous
objectives, methodology, participants (age, staging, timing of ART, CNS
drug penetration) and psychometric testing [7]. There is still paucity
of data from LMICs – the available Indian data is restricted to older
children and adolescents [8,9]. As ART policy changes and improving
health care translates into more survivors, there is a need to detect
potential developmental or mental health issues in childhood, for timely
intervention.
This study compared development/cognition, adaptive
function and maladaptive behavior of perinatally-acquired HIV-infected
and HIV-uninfected exposed pre-school and school-aged children with
HIV-uninfected (HU) controls. Their correlation with specific clinical
and environmental factors was determined.
Methods
This hospital-based cross-sectional study was
conducted from November, 2013 to March, 2015, after obtaining
Institutional ethics committee approval. Cases included children aged
2-9 years who were either HIV-infected (seropositive on three
consecutive ELISA rapid tests) or HIV-exposed but uninfected (HIV
negative siblings, or children born to seropositive patients), recruited
from the ART centre of our hospital. Children of the same age-group,
presenting with minor illnesses, were enrolled as controls and
considered to be HIV-uninfected. Exclusion criteria were significant
perinatal events requiring hospitalization, previous neurological
infections, and known neurodevelopmental or chronic disorders.
Sequential enrollment continued till a pre-decided sample size of
convenience (based on ART center attendance of the previous year) was
attained. Parental informed consent was obtained; however, assent of
older children was not taken to avoid inadvertent disclosure.
Locally developed, provider-completed, Hindi
translations of Developmental profile-3 (DP 3) [10], Vineland Adaptive
Behaviour Scale, 2 nd edition
(VABS 2) [11] and Child Behavior Checklist (CBCL) [12] were administered
by a trained pediatrician. The parental questionnaire DP 3 assesses
physical, adaptive, social-emotional, cognitive and communication
domains from birth to 12 years. It computes standard scores (SS) for
domains and an overall General Development Score (GDS), which are rated
as Delay, Below average and Average. GDS represents development in
children younger than five years and cognition in older children. VABS 2
is a parental interview that measures adaptive function in
socialization, daily living skills, communication, and motor domains.
Adaptive Behavior Composite (ABC) and SS rate overall and domain-wise
performance as Low, Moderate low, and Adequate. Maladaptive Behavior
Index and Maladaptive Critical Index is assessed in children
ł3 years. CBCL is a
parental interview that categorizes behavior and rates them as Normal,
Borderline and Clinical, based on standardized T scores.
History, examination and records were reviewed after
which psychometric tests were scored. Primary outcome measures included
significant impairment in development/cognition (delay), adaptive
function (low) or maladaptive behavior (significant MBI, MCI or T
scores).
Statisticial analyses: SPSS version 20.0 was used
for statistical analyses. Unpaired t-test and Chi square test were used
for inter-group comparisons. Pearson’s coefficient of correlation (r)
was determined by univariate analysis between individual clinical
(staging, nutritional status, CD4 status, ART, central nervous system
(CNS) penetration) and environmental covariates (socio-economic status,
family composition, parental literacy, HIV and ART status, and death)
and primary outcomes (VABS-2, DP-3 and CBCL scores). Since development
is affected by the cumulative effect of multiple factors, multivariate
analysis was applied between combined clinical and environmental
covariates with the primary outcomes. Strength of association was
measured by multiple correlation coefficient (R).
Results
Of the 113 eligible children, 8 were excluded (3,
epilepsy; 4, significant perinatal events; 5, developmental disorders;
and 1, meningitis). The study population comprised of 50 HIV-infected
children (HI group), 25 HIV-exposed uninfected children (HEU group) and
25 HIV-uninfected (HU group). The mean (SD) age was 4.9 (2.2), 6.1
(1.9), 5.1 (2.2) years and male: female ratio 2.8, 1.1 and 1.1,
respectively in the three groups. Table I displays
baseline participant details in the three groups. Distribution of WHO
clinical staging in HI group was 24 (48%) T1, 12 (24%) T2, 8 (16%) T3
and 6 (12%) T4; 14 (28%) had CD4 percentage <25% while 15 (30%) had CD4
counts <350µl. Forty five (90%) were on ART; 22 (48.9%) with high CNS
penetration and 23 (51.1%) medium CNS penetration. Mean (SD) duration of
ART was 1.2 (0.9) years in children aged 2-5 years and 2 (2) years in
those between 5 and 9 years. None had microcephaly or focal neurological
signs.
TABLE I Baseline Characteristics of Children Enrolled in the Study (N=100)
|
Characteristics |
|
HI (n=50) |
HEU (n=25) |
HU (n=25) |
|
Parental education |
Mother ≤ primary
|
27 (54) |
20 (80) |
7(28) |
|
Father ≤ primary
|
10 (20)
|
8 (32) |
7 (28) |
|
Child education |
No informal (<5 y) |
23/30 (77.7) |
5/7 (71.4) |
7/11 (63.6) |
|
No school (>5 y) |
7/20 (35) |
14/18 (77.7) |
11/14 (78.5) |
|
#Socioeconomic status (SES) |
Lower middle
|
11 (22) |
11 (44) |
9 (36) |
|
Upper lower |
36 (72) |
14 (56) |
12 (48) |
|
Family status |
Nuclear family
|
31 (62) |
20 (80) |
23 (92) |
|
Maternal death |
4 (8) |
1 (4) |
0 |
|
Paternal death |
11 (22) |
2 (8) |
0 |
|
Both death |
4 (8) |
1 (4) |
0 |
|
Anti-retroviral therapy |
Antenatal |
7 (14) |
3 (12) |
- |
|
Maternal |
36 (85.7) |
6 (14.2) |
- |
|
Paternal |
30 (88.2) |
9 (26.5) |
- |
|
Nutritional status |
Severe stunting (<5 y) |
19/30 (63.3) |
0/11 |
0/7 |
|
Severe thinness (>5 y) |
1/20 (5) |
3/18 (16.7) |
4/14 (28.6) |
|
HIV: Human Immunodeficiency, HEU: HIV-exposed uninfected,
HI:HIV-infected, HU: HIV-uninfected; Significant differences (P
<0.05) noted in the following intergroup comparisons:
P<0.05 for children not attending school, Lower Middle SES,
parents on ART and severe s between HI and HEU, Maternal
education, children not attending school, Upper lower SES,
nuclear families and severe stunting – between HI and HU; and
maternal education between HEU and HU; #Using modified
Kuppuswamy socioeconomic status scale (1 child each in Upper
strata in HI and HU groups, and 2 and 3 children in Upper middle
strata in HI and HU groups, respectively). |
Significant development/cognitive impairment was
observed in 38 (76%) HI, 16 (64%) HEU, and 6 (24%) (SD) HU children.
Mean (SD) GDS of HI and HEU children was significantly lower than HU;
59.2 (16.9), 70.1(13.4) and 77.4(13.4), respectively. Significant
adaptive impairment was seen in 12 (24%) HI and 2 (8%) of HEU children.
Mean (SD) ABC of HI group was significantly lower than HEU group and HU
group [75.3 (8.9) vs 85.5 (7.3), P<0.05].
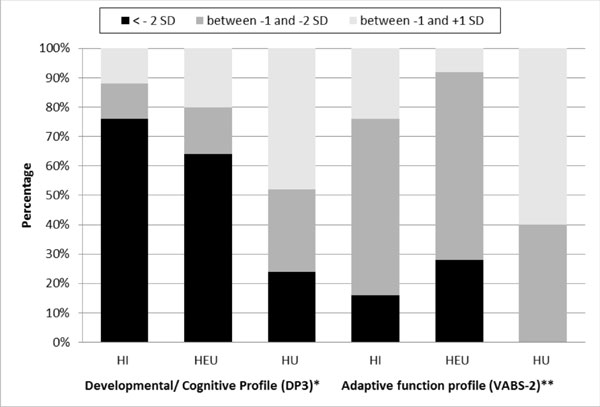 |
|
Fig. 1 Distribution of levels of
performance of development/cognition and adaptive function in
HIV-infected (HI), HIV-exposed and uninfected (HEU) and
HIV-uninfected (HU) groups.
|
Distribution of levels of development/cognition and
group [75.3 (8.9) vs 85.4 (7.3), P<0.05] adaptive function
is depicted in Fig. 1, and domain-wise performance in
Web Table I. Significant maladaptive behavior was not
observed in any group. Mean (SD) MBI was 11.7 (1.9), 11.4 (1.1) and 11.2
(1.1) in the HI, HEU and HU group, respectively. The mean (SD) CBCL T
score of HI group [29.2 (6.1)] was significantly higher than HEU group
[26.0 (1.7)] and HU [26.6 (1.8)] children, but not clinically relevant.
No single independent clinical or environmental
covariate had a strong correlation with GDS, ABC or T scores in HI
group. Combined clinical covariates displayed strong and significant (P<0.05)
association with GDS (R=0.84), ABC (R 0.81) and T scores (R 0.73).
Association between combined environmental covariates and GDS was strong
in HI (R=0.73, P<0.05) and HEU (R=0.74, P<0.05), moderate
with ABC in HI (R=0.59, P<0.05) and HEU (R=0. 66, P<0.05),
and weak with ABC in HU (R= 0.45, P<0.05).
Discussion
We found that the group as a whole had poor maternal
literacy, low pre-school/ school attendance, and thinness. A strong and
significant association was found between combined environmental factors
and overall develop-ment (GDS). The high prevalence of impairment in our
controls is less than the estimated 43% of children under five years
from LMIC who are not expected to attain their developmental potential
[13].
Significant cognitive impairment and low scores in
developmental and adaptive function was seen in HIV-infected children.
Studies from LMIC [4,5,14] have reported this earlier, attributing it to
delayed ART (more severe and pervasive neurological damage) and adverse
ecological and biological factors [15]. Neurodevelop-mental outcomes are
better when ART is started in infancy, as it decreases irreversible CNS
damage [5]. Though 90% of children in this study were receiving ART,
initiation in infancy had not been universal due to the then existing
ART policy. Adaptive function in HI children is affected by co-morbid
illnesses, increased dependence on caregivers, decreased opportunities,
and poor parental support, besides CNS dysfunction [16]. This explains
the significant differences between HIV-infected and HIV-exposed
uninfected children with respect to proportion, severity of impairment
and strength of association between environmental factors and ABC.
Children in HEU group had more cognitive impairment
than controls, and a strong association with combined environmental
covariates. This was also observed by Bass, et al. [2] in Ugandan
children. Surprisingly, few studies from high-income countries report
equal or even higher impairment in HEU group than HIV-infected children
[8]. This phenomenon is explained by HI group children receiving high
quality medical, nutritional, and social support, while HIV-exposed but
uninfected counterparts remain unsupported. The increased maladaptive
behavior reported in older HIV- infected children and adolescents
[8,16-19] is attributed to psychological responses to debilitating
illness and disclosure. The absence of maladaptive behavior in our study
may be attributed to less severe disease in the majority, non-disclosure
and good medical care (90% on ART, good adherence to therapy,
nutritional support and regular follow-up), all of which promote
resilience [4].
Though the Hindi translations of the psychometric
tools have not been validated in Indian community, they are routinely
used in clinical practice and research. The lack of Indian norms was
addressed by including a demographically-matched control group. Other
limitations were small sample size (being single centric) and lack of
blinding. An attempt was made to decrease information bias by
interpreting the tests after collection of clinical data. An adequately
powered, multi-centric, longitudinal study with age stratification is
recommended to provide deeper insight.
In India, ART became universally available to all
HIV-infected children after a policy change in 2014 [20]. Its clinical
implications are earlier initiation of ART and more children surviving
till adolescence and adulthood. Though biological HIV-related risk
factors may decrease, the ecological adverse influence will remain with
their associated impact of cognition, adaptive function and behavior. We
suggest that the National AIDS Control Organization consider
incorporating their assessment in standard care of all HIV-exposed
children, whether infected or not.
Contributors: SBM,AS: conceptualized the
study and will stand as guarantors; SD,SBM: did the literature search;
SD: was trained to administer the psychometric tools by SBM and SS and
collected the data; SBM,SD,SS: interpreted and analyzed the data; SM:
drafted the manuscript which underwent a critical appraisal by SD,AS,SS.
All the authors approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Funding: None; Competing interests:
None stated.
|
What This Study Adds?
• Impairment in development/cognition and adaptive
function was found in HIV-infected and HIV-exposed
uninfected children aged 2-9 years.
|
References
1. Fiegelman S. Overview of assessment and
variability: Growth, development and behavior. In: Kliegmen RM,
Stanton BF, St Geme III JW, eds. Nelson Textbook of Pediatrics.
20th ed. Philadelphia: Elsevier; 2016. p. 48-51.
2. Bass JK, Nakasujja N, Familiar-Lopez I, Sikorskii
A, Murray SM, Opoka R, et al. Association of caregiver quality of
care with neurocognitive outcomes in HIV affected children aged 2-5
years in Uganda. AIDS Care. 2016;28:76-83.
3. Coscia JM, Christensen BK, Henry RR, Wallston K,
Radcliffe J, Rutstein R. Effects of home environment, socioeconomic
status, and health status on cognitive functioning in children with
HIV-1 infection. J Pediatr Psychol. 2001;26:321-9.
4. Smith R, Chernoff M, Williams PL, Malee KM, Sirios
PA, Kammerer B, et al. Impact of HIV severity on cognitive and
adaptive functioning during childhood and adolescence. Pediatr Infect
Dis J. 2012;31:592-8.
5. Puthanakit T, Aurpibul L, Louthrenoo O, Tapanya P,
Nadsasarn R, Insee-ard S, et al. Poor cognitive functioning of
school-aged children in Thailand with perinatally acquired HIV infection
taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24:141-6.
6. Brahmbhatt H, Boivin M, Ssempijja V, Kigozi G,
Kagaayi J, Serwadda D, et al. Neurodevelopmental benefits of
Anti-Retroviral Therapy in Ugandan children 0–6 Years of age with HIV. J
Acquir Immune Defic Syndr. 2014;67:316-22.
7. Laughton B, Cornell M, Boivin M, Van-Rie A.
Neurodevelopment in perinatally HIV-infected children: a concern for
adolescence. J Int AIDS Soc. 2013;16:18603.
8. Grover G, Pensi T, Banerjee T. Behavioural
disorders in 6-11-year-old, HIV-infected Indian children. Ann Trop
Paediatr. 2007;27:215-24.
9. Joshi D, Tiwari MK, Kannan V, Dalal SS, Mathai SS.
Emotional and behavioural disturbances in school going HIV positive
children attending HIV clinic. Med J Armed Forces India. 2017;73:18-22.
10. Gerald D. Developmental Profile, 3rd ed. Los
Angeles, CA: Western Psychological Services; 2007.
11. Sparrow S, Cicchetti DV, Balla D. Vineland
adaptive behavior scale, 2nd ed. San Antonia: Pearson Assessment; 2005.
12. Achenbach TM, Rescorta LA. Manual for the ASEBA
school age forms and profiles. Burlington, VY: University of Vermont;
2001.
13. Black MM, Walker SP, Fernald LCH, Anderson CT,
DiGirolomo AM, Chunling L, et al. Early childhood development
coming of age: Science through the life course. Lancet. 2017;389:77-90.
14. Nozyce M, Lee S, Wiznia A, Nachman S, Mofenson L,
Smith M, et al. A behavioral and cognitive profile of clinically
stable and HIV infected children. Pediatrics. 2006;117:763-70.
15. Boyede GO, Lesi FEA, Ezeaka VC, Umeh CS. The
influence of clinical staging and use of antiretroviral therapy on
cognitive functioning of school aged Nigerian children with HIV
infection. J AIDS Clin Res. 2013;4:195.
16. Malee KM, Tassiopoulos K, Huo Y, Siberry G,
Williams PL, Hazra, R, et al. Mental health functioning among
children and adolescents with perinatal HIV infection and perinatal HIV
exposure. AIDS Care. 2011;23:1533-44.
17. Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner
SL, Hazra R, Civitello L. Cognitive functioning in school aged children
with vertically acquired HIV infection being treated with Highly active
antiretroviral therapy (HAART). Dev Neuropsychol. 2006;30:633-57.
18. Gadow KD, Chernoff M, Williams PL, Brouwers P,
Morse E, Heston J. Co-occurring psychiatric symptoms in children
perinatally infected with HIV and peer comparison sample. J Dev Behav
Pediatr. 2010;31:116-28.
19. Gosling A, Burns J, Hirst F. Children with HIV in
the UK: A longitudinal study of adaptive and cognitive functioning. Clin
Child Psychol Psychiatry. 2004;9:25-37.
20. National AIDS Control Organization. NACO Annual
Report 2013-14. Available from:
http://naco.gov.in/documents/annual-reports. Accessed
December 27, 2015.
|
|
|
 |
|

