|
|
|
Indian Pediatr 2019;56: 929-932 |
 |
Correlation of Dickkopf-1 with Inflammation
in Crohn Disease
|
|
Mi Jin Kim1,2 and Yon Ho Choe1
From 1Department of Pediatrics, Samsung Medical
Center, Sungkyunkwan University School of Medicine, Seoul; and 2Chungnam National
University Hospital, College of Medicine, Chungnam National University,
Daejeon; Korea.
Correspondence to: Mi Jin Kim, Department of
Pediatrics, Samsung Medical Center, Sungkyunkwan University School of
Medicine, 81 Irwon-ro, Gangnam-gu, Seoul, 06351, Korea.
Email:
[email protected]
Received: March 14, 2019;
Initial review: April 16, 2019;
Accepted: September 03, 2019.
|
|
Objective: To explore the
potential roles of Dickkopf-1 (DKK-1) and
b-catenin
in Crohn disease, and to evaluate the effects of a tumor necrosis factor
(TNF)-a
inhibitor on Wnt signaling in patients with the disease. Methods:
We enrolled 21 patients who received infliximab treatment for one year
and achieved clinical remission during the treatment period. Disease
activity was graded according to the Pediatric Crohn’s Disease Activity
Index (PCDAI). Peripheral blood and colonic mucosal specimens were
collected from all patients with Crohn disease and from 14 healthy
controls. DKK-1 levels in serum were detected by enzyme-linked
immunosorbent assay (ELISA). Total RNA for DKK-1 and
b-catenin
from the frozen colonic tissue were obtained via real-time
quantitative reverse transcription-polymerase chain reaction (RT-PCR).
Serum C-reactive protein (CRP) levels, erythrocyte sedimentation rates
(ESR), and albumin were also measured in patients with Crohn disease
before and after infliximab therapy. Results: The serum levels of
DKK-1 were significantly higher in patients with Crohn disease than in
healthy controls (P=0.003) and were decreased in those treated
with infliximab (P=0.026). Serum DKK-1 level was correlated with
levels of ESR (r=0.527, P=0.025), CRP (r=0.502, P=0.034),
albumin (r=0.363, P=0.021) and PCDAI (r =0.462, P=0.054)
in Crohn disease. DKK-1 mRNA expression in the colonic mucosa was higher
in patients than in controls and decreased after infliximab treatment.
b-catenin
expression in the colonic mucosa was lower in patients than in controls
and increased after infliximab treatment. However, the differences were
not significant (P>0.05). Conclusions: DKK-1 might be an
important mediator of the pathogenesis of Crohn disease, and changes in
DKK-1 levels may serve as biomarkers of inflammation in these patients.
Keywords:
b-catenin,
Infliximab, Pathogenesis, Wnt signaling.
|
|
C rohn disease (CD) is a
multifactorial disease of unknown etiology characterized by a chronic
inflammation of the entire gastrointestinal tract, most commonly
occurring in the distal ileum and colon [1]. Immune, genetic, and
environmental factors are thought to contribute to CD [2]. Cytokine
tumor necrosis factor (TNF)-a
contributes substantially to the pathology of Crohn disease, a role that
is highlighted by the responsiveness of the disease to TNF-a
blocking agents [3].
Signaling by the Wnt family of secreted lipoproteins
plays a central role in embryogenesis and tissue homeostasis [4,5].
Abnormal Wnt/ b-catenin
signaling is associated with many diseases in humans, including cancer
and osteoporosis [5,6]. b-catenin
is a fundamental component of the Wnt signaling pathway, which acts as a
coactivator through its ability to recruit components that promote
chromatin remodeling and the transcriptional process [7]. Dickkopf-1
(DKK-1) is a secreted glycoprotein that has been shown to act as a
potent inhibitor of the canonical Wnt/b-catenin
signaling pathway [8,9]. DKK-1 plays essential roles in many biological
processes, ranging from the induction of anterior mesoderm formation and
head development during embryogenesis to bone formation and bone mass
regulation in adult organisms [10]. However, relatively little is known
about the localization of Wnt signaling components and the importance of
DKK-1 within the intestine.
The Wnt signaling pathway is associated with
regulation of homeostasis within the colonic stem cell compartment,
controlling the balance between proliferation and differentiation
[11,12]. Questions regarding whether DKK-1 is associated with
inflammation in Crohn disease, or whether there are correlations between
DKK-1 and clinical and laboratory characteristics, remain unanswered (Web
Fig. 1).
In this study, we explored the potential roles of
DKK-1 and b-catenin
in CD and evaluated the effects of a TNF-a
inhibitor (infliximab) on Wnt signaling in patients with CD.
Methods
From among pediatric patients who were diagnosed with
Crohn disease in accordance with the European Society for Pediatric
Gastroenterology, Hepatology and Nutrition - Porto criteria [13] at the
Samsung Medical Center between January 2010 and August 2013, we enrolled
21 patients who received infliximab treatment for one year and who
achieved clinical remission during the treatment period. Disease
activity was graded according to the Pediatric Crohn’s Disease Activity
Index (PCDAI) [14]. We recruited 14 children with macroscopically and
histologically normal mucosa and no evidence of any underlying
gastrointestinal conditions as healthy controls.
A monoclonal immunoglobulin G1 chimeric antibody
directed against TNF- a
(infliximab) was administered to CD patients by intravenous infusion at
a dose of 5 mg/kg at weeks 0, 2, and 6, and this course was repeated
every 8 weeks for 10 months thereafter.
This study was approved by the Institutional Review
Board of the Samsung Medical Center. All participants provided written
informed consent before participation in this study.
Peripheral blood samples were collected to assess
DKK-1 levels by enzyme-linked immunosorbent assay (ELISA) from all Crohn
disease patients and healthy control individuals. In patients with Crohn
disease, blood collection was performed before infliximab therapy and
after the eighth course of infliximab therapy. Serum C-reactive protein
(CRP) levels, erythrocyte sedimen-tation rates (ESR), and albumin were
also measured in patients with Crohn disease before and after infliximab
therapy.
Colonic mucosal specimens from all 21 CD patients and
14 healthy controls were assessed for mRNA expressions of DKK-1 and
b-catenin.
Colonoscopy was performed for all patients by a single pediatric
gastroenterologist, who collected one or two additional biopsies within
close proximity of the area in which biopsies were taken for routine
histology. In patients with Crohn disease, mucosal biopsies were
performed before infliximab therapy and after eight courses of
infliximab therapy. The specimens were frozen and stored at -80°C for
RNA isolation.
DKK-1 levels were determined in serum samples from
Crohn disease patients and healthy controls using a commercially
available LINCOplex kit (Millipore, Billerica, MA, USA) and a Luminex
analyzer, according to the manufacturer’s instructions. The result was
calculated through the Bio-Plex Manager Software (Bio-Rad Laboratories,
Hercules, CA, USA) and the cytokine concentration in plasma was
expressed as pg/mL.
Total RNA for DKK-1 and
b-catenin from the
frozen colonic tissue was obtained using Trizol reagent (Invitrogen)
according to the manufacturer’s instructions. The amount and purity of
the obtained RNA was determined using a ND-1000 spectrophotometer (Nanodrop
Technologies, Wilmington, DE, USA). Reverse transcription was performed
using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen).
cDNA was prepared from 1 µg of mRNA with oligo/dT according to the
manufacturer’s instructions. Real-time PCR was constructed using
commercially available assays for the DKK-1 gene (assay ID
Hs00183740_mL, Genbank accession number NM_012242.2; Applied Biosystems,
Foster City, CA, USA), b-catenin
(assay ID Hs01076483_mL, Genbank accession number NM_001904.3), and
human endogenous control GAPDH (assay ID Hs99999905_mL, Genbank
accession number NM_002046.3) in combination with the TaqMan Universal
PCR Master Mix (Applied Biosystems). Then, PCR was performed in a 7900HT
real-time PCR system (Applied Biosystems). Comparative analyses of each
gene were performed using computer programs SDS 2.3 and RQ 2.1 (Applied
Biosystems). The relative gene expressions (RQ) were calculated using
the 2_ŃŃCT
method.
Statistical analyses: Statistical analyses were
performed using the Mann-Whitney U-test for unpaired samples and the
Wilcoxon signed-rank test for paired samples using SPSS 24.0 (SPSS,
Chicago, IL, USA). Correlations between DKK-1 or
b-catenin expression
from CD patients and disease activity were determined by simple linear
regression. Values of P<0.05 were considered statistically
significant.
Results
The enrolled children included 21 with Crohn disease
(16 males) and 14 healthy controls (12 males). The mean age of the
healthy controls was patients with Crohn disease did not differ
significantly [15.9 (1.4) vs 14.4 (2.1) years]. After one year of
infliximab treatment, disease activity according to PCDAI score, and
from ESR and C-reactive protein declined significantly (Table
I).
TABLE I Baseline Characteristics and Clinical Outcomes
|
Controls |
Patients (n=21) |
|
(n=14) |
Before IFX Tx |
After IFX Tx |
|
Albumin |
4.7 (0.2) |
3.9 (0.4) |
4.5 (0.3) |
|
ESR (mm/hr) |
11.1 (11.8) |
61.9 (31.0) |
16.1 (14.2) |
|
CRP (mg/dL) |
0.6 (1.8) |
2.1 ( 2.2) |
0.2 (0.3) |
|
PCDAI score |
|
35.2 (10.8) |
4.2 (4.9) |
|
IFX Tx: infliximab therapy; ESR: Erythrocyte sedimentation
rate; CRP: C-reactive protein; PCDAI: Pediatric Crohn Disease
Activity Index. |
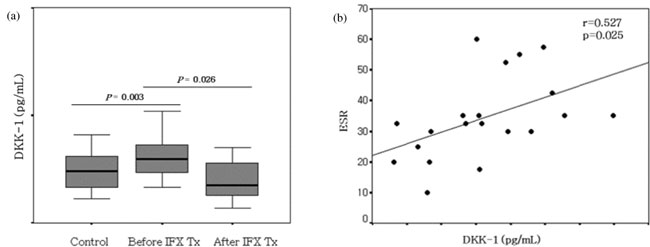 |
|
Fig. 1 Expression of Dickkopf-1 in
peripheral blood. (a) The serum levels of
Dickkopf-1 were significantly higher in patients with Crohn
disease than in healthy controls (P=0.003) and were decreased in
Crohn disease patients treated with infliximab (P=0.026). (b)
Serum Dickkopf-1 levels were correlated with levels of ESR
(r=0.527, P=0.025) in patients with Crohn disease.
|
The plasma level of DKK-1 in CD patients before
infliximab therapy was significantly higher than that of healthy
controls (Fig. 1a, P=0.003). After infliximab
therapy, the level of DKK-1 in CD patients was decreased compared to
that before therapy (Fig. 1a, P=0.03). Correlations
were found between DKK-1 in plasma from children with Crohn disease and
ESR (r=0.527, P=0.025), CRP (r=0.502, P=0.034), albumin
(r=0.363, P=0.021) and PCDAI (r=0.462, P=0.054) (Fig.
1b).
DKK-1 mRNA expression in the colonic mucosa was
higher in patients than in controls [2.18 (1.69) vs 1.60 (1.48)]
and decreased after infliximab treatment [2.18 (1.69) vs 1.83
(2.32)]. b-catenin
expression in the colonic mucosa was lower in patients than in controls
[0.69 (1.19) vs 1.02 (1.25)] and increased after infliximab
treatment [0.69 (1.19) vs 0.92 (0.71)]. However, there were no
significant differences between each groups before and after infliximab
therapy or compared to normal controls. There were no correlations
between DKK-1 or b-catenin
in the colonic mucosa from children with Crohn disease and disease
activity.
Discussion
Since Wnt signaling is known to cause cancer and
malformations, many animal models are now in use to assess the
pathogenesis of various diseases and the toxicity of therapeutic agents.
Recently, a number of diseases have been associated with abnormalities
in Wnt signaling, including adipogenesis [15], schizophrenia [16], and
Alzheimer disease [17], as well as cancer and developmental
difficulties. Therefore, the role of Wnt signaling as a pathogenic
process in various diseases has been examined in the context of efforts
to identify the role of Wnt signaling in cancer. DKK-1 is a downstream
target gene for Wnt/ b-catenin
signaling, and has been shown to regulate Wnt signaling through negative
feedback [18,19]. Inflammatory stimuli such as TNF-a
induce DKK-1 release in various cells [20]. Inflammatory bowel disease
can lead to chronic relapsing inflammation of the gastrointestinal
tract. The Wnt antagonist DKK-1 is induced by inflammatory cytokines and
that exacerbates intestinal inflammation. However, little is known about
the localization of Wnt signaling components and the importance of DKK-1
in inflammatory bowel disease, especially CD.
In this study, we found that DKK-1 was inhibited by
TNF- a
inhibition in CD patients. The plasma level of DKK-1 in CD patients
before infliximab therapy was significantly higher than that of healthy
controls. After infliximab therapy, the level of DKK-1 in CD patients
was decreased compared to that before therapy. Correlations were also
found between DKK-1 in plasma from CD patients and inflammatory marker
or disease activity. Inhibition of DKK-1 may increase the
transcriptional activity and survival signaling pathway of
b-catenin, thereby
promoting epithelial cell proliferation and wound repair. These results
suggest that the Wnt signaling activator can be used to maintain the
regeneration of gut epithelium, homeostasis, and to treat CD.
The number of patients was relatively low, which
requires a cautious interpretation of the results. Preventive and
therapeutic Wnt/ b-catenin
activation led to a significant improvement of CD. Future in vitro
and in vivo studies will certainly provide more insights into the
principles underlying decreased Wnt/b-catenin
signaling in CD, and whether therapeutic Wnt/b-catenin
activation will present as a future therapeutic tool in this chronic
disease.
Since DKK-1, as a part of the canonical Wnt/ b-Catenin
pathway, has already been shown to be involved in pathological processes
such as cell migration and invasion, this observation also supports the
hypothesis that DKK-1 might be involved in the pathogenesis of CD
patients. DKK-1 may be an important mediator in the pathophysiology of
CD, and DKK-1 level may be a good marker for predicting the inflammation
and prognosis of CD patients. Our findings warrant further research
examining the potential of DKK-1 as a therapeutic agent of CD.
Contributors: Both authors have contributed,
designed and approved the final version of manuscript, and are
accountable for all aspects related to the study.
Funding: This work was supported by the research
fund of Chungnam National University.
Competing interests: None stated.
|
What this Study Adds?
•
Dickkopf-1 (DKK-1) may be a mediator in the pathophysiology
of Crohn disease.
•
DKK-1 level are higher with increasing inflammation and
decrease with treatment in children with Crohn disease.
|
References
1. Podolsky DK. Inflammatory bowel disease. N Engl J
Med. 2002;347:417-24.
2. Xavier RJ, Podolsky DK. Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-34.
3. Papadakis KA, Targan SR. Role of cytokines in the
pathogenesis of inflammatory bowel disease. Annu Rev Med.
2000;51:289-98.
4. Logan CY, Nusse R. The Wnt signaling pathway in
development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810.
5. Clevers H. Wnt/beta-catenin signaling in
development and disease. Cell. 2006;127:469-80.
6. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and
beta-catenin signalling: diseases and therapies. Nat Rev Genet.
2004;5:691-701.
7. Willert K, Jones KA. Wnt signaling: is the party
in the nucleus? Genes Dev. 2006;20:1394-404.
8. Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki
T, Bottaro DP, et al. Isolation and biochemical characterization
of the human Dkk-1 homologue, a novelinhibitor of mammalian Wnt
signaling. J Biol Chem. 1999;274:19465-72.
9. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock
C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins
and functions in head induction. Nature. 1998;391:357-62.
10. Niehrs C. Function and biological roles of the
Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469-81.
11. de Lau W, Barker N, Clevers H. WNT signaling in
the normal intestine and colorectal cancer. Front Biosci.
2007;12:471-91.
12. Pinto D, Clevers H. Wnt control of stem cells and
differentiation in the intestinal epithelium. Exp Cell Res.
2005;306:357-63.
13. Inflammatory bowel disease in children and
adolescents: Recommendations for diagnosis-the Porto criteria. J Pediatr
Gastroenterol Nutr. 2005;41:1-7.
14. Hyams JS, Ferry GD, Mandel FS, Gryboski JD,
Kibort PM, Kirschner BS, et al. Development and validation of a
pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr.
1991;12:439-47.
15. Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati
N, Johnson KW, et al. Regulation of Wnt signaling during
adipogenesis. J Biol Chem. 2002;277:30998-1004.
16. Miyaoka T, Seno H, Ishino H. Increased expression
of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38:1-6.
17. Jaworski T, Dewachter I, Lechat B, Gees M, Kremer
A, Demedts D, et al. GSK-3alpha/beta kinases and amyloid
production in vivo. Nature. 2011;480:E4-5; discussion E6.
18. Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura
Y, Suzuki Y, et al. DKK1,anegative regulator of Wnt signaling, is
a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520-6.
19. Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco
N, Pena C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a
downstream target of beta-catenin/TCF and is downregulated in human
colon cancer. Oncogene. 2005;24:1098-103.
20. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky
MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint
remodeling. Nat Med. 2007;13:156-63.
|
|
|
 |
|

