|
|
|
Indian Pediatr 2019;56:913-916 |
 |
Effect of Different
Doses of Inhaled Corticosteroids on the Isolation of
Nasopharyngeal Flora in Children with Asthma
|
|
Garima Nirmal 1,
Shally Awasthi1,
Sarika Gupta1 and
Jyotsna Aggarwal2
From Depatments of 1Pediatrics, and 2Microbiology,
King George Medical University, Lucknow, Uttar Pradesh, India.
Correspondence to: Dr Shally Awasthi, Department of Pediatrics, King
George Medical University, Lucknow 226 003, Uttar Pradesh, India.
Email: [email protected]
Received: September 13, 2018;
Initial review: February 19, 2019;
Accepted: September 04, 2019.
|
Objectives: To find the effects of inhaled corticosteroids and the
impact of different doses of inhaled corticosteroids on the isolation of
nasopharyngeal flora in asthmatic children aged 1-15 years. Methods:
The study included 75 children with asthma and 25 age-matched controls.
Nasopharyngeal swabs were obtained. Bacteria were identified by standard
techniques. Results: Pathogenic organisms were isolated from 36%
of asthmatic children and 20% of controls, the difference was not
significant statistically (OR=2.25, 95% CI=0.75-6.67, P=0.13).
There was no statistically significant association of using a high dose
of inhaled corticosteroids with the isolation of pathogenic organisms.
Usage of biomass fuel for cooking in the household of asthmatic children
increases the risk of colonization (OR=3.4, 95% CI= 1.26-9.10, P=0.03).
Conclusion: Inhaled corticosteroids are safe in the treatment of
asthma and there is no association between different doses of Inhaled
corticosteroids and isolation of the pathogenic organism.
Keywords: Biomass fuels, Management, Pneumococcus.
|
|
I
nhaled corticosteroids (ICS) form the cornerstone
of treatment of asthma. Local side-effects associated with ICS use
include oropharyngeal candidiasis, dysphonia, reflex cough, bronchospasm,
and pharyngitis due to the weakening of local immunity [1]. In children,
the nasopharyngeal flora flora becomes established within the first 12
months of their life including both commensal bacteria and potential
pathogens such as Streptococcus pneumoniae, Haemophilus
influenza [2].
Deposition of ICS in the oropharynx may alter the
local mucosal immune response through their immunosuppressive effects
which have been considered responsible for oropharyngeal candidiasis
[3]. Arocha-Sandoval, et al. [4] found a higher rate of
oropharyngeal bacterial colonization in asthmatic children as compared
with healthy individuals. As colonization with potential pathogens can
lead to the development of respiratory or even invasive infections,
recognition of the risk factors for such colonization is important.Thus,
the main objective of the study was to investigate the effects of
steroids on bacterial colonization and to analyze the impact of
different doses of ICS on nasopharyngeal isolation of a pathogenic
organism.
Methods
The study was carried out over a period of one year
(September 2015 to July 2016) in the outpatient department of
Pediatrics, and department of Microbiology of a tertiary-care referral
teaching Institute of Northern India after obtaining ethical clearance
from the institutional ethics committee.
All diagnosed cases of asthma according to GINA
guidelines were enrolled [5]. We excluded the children who received
antibiotics or got hospitalized in the last 15 days. For children aged
1-5 years, spirometry was not possible. Thus, cases were recruited that
had a presence of two or more of the following symptoms: current
presence of wheeze in any child with a history of more than two episodes
of documented wheeze or use of bronchodilator in the preceding 12
months; on any regular medication for asthma such as corticosteroids,
b-2 agonist,
methylxanthines, leukotriene modifiers, and cromones; and, presenting
with symptoms of asthma along with positive family history of asthma or
other allergic disease (allergic rhinitis or eczema).
Controls were enrolled from the children attending
the immunization clinic or siblings of children attending the OPD, from
the same locality/ community for some other ailments. Inclusion criteria
for controls included no past or present diagnosis of asthma and other
pulmonary diseases; no history of wheezing, shortness of breath, and
other symptoms of allergic diseases such as nasal and skin symptoms; no
use of immunosuppressant or medications for asthma and, absence of
first-degree relatives with a history of asthma.
Almost all the patients were taking inhaled
budesonide in our study, only two patients used inhaled fluticasone. A
dose of 100-200 µg was considered as low dose, >200-400 µg was
considered as moderate dose and >400 µg dose was considered as a high
dose of inhaled budesonide in the children aged 6-11 years while the
children who were 12 years and older, an inhaled dose of 200- 400 µg,
>400-800 µg, and >800 µg were considered as low dose, moderate dose, and
high dose, respectively [5]. A predesigned data collection form was
filled and a nasopharyngeal swab was taken. After obtaining the consent
and explaining the procedure to parents and child, the patient’s head
was tilted back to 70 degrees. The distance from ala of the nose to
tragus was measured and marked on the swab. The swab was inserted
horizontally into nostril up to at a point equivalent to half the
distance measured or until resistance is met. The swab was rotated and
hold in place for 5-10 seconds. Tip of the swab was placed into a
sterile tube and immediately transported to the laboratory (transport
media was not used).
The swab was cultured on sheep blood agar, Mc Conkey
agar and chocolate agar and a direct smear were prepared and the gram
stain was made. Plates were immediately incubated at 37°C in 5% CO 2
incubator for overnight. After that, culture growth was reported and
colonies were identified. Colony morphology was identified as per
standard protocol [6]. The bacteria were divided into two groups:
potentially pathogenic bacteria mainly S. aureus, S. pneumoniae, M.
catarrhalis and gram-negative rods like Acinetobacter, Enterobacter
and E. coli. Bacteria other than them were included in
non-pathogenic group/commensals, which mainly includes Coagulase-negative
S. aureus, S. viridans, and Diptheroids. Subjects in whom, both
potentially pathogenic and nonpathogenic bacteria were present, were
included in the potentially pathogenic group.
Statistical analysis: The analysis was performed
on SPSS software (Windows version 17.0) and Epi Info 7. Categorical
groups were compared by the chi-square (-2) test and Fisher exact test.
We calculated odd’s odds ratio with a 95% confidence interval. A
two-tailed P-value less than 0.05 was considered statistically
significant.
Results
Out of 86 patients screened, 11 patients were
excluded as per the exclusion criteria. Included were 75 asthma cases
and 25 age-matched healthy controls. The baseline characteristics of the
study population are given in Table I. There was a
statistically significant difference between the smoking status of
father and the usage of biomass fuel for cooking among cases and
controls. Pneumococcal vaccines were taken by 25.3% of asthmatic
children and 20% of controls (information on influenza vaccine was not
collected). Among recruited cases, 40 (53.3%) had well controlled, 23
(30.7%) had partially controlled and 12 (16%) had uncontrolled asthma.
Out of 75 children with asthma, 33 (44%), 33 (44%) and 9 (12%) were
using low dose, moderate dose and the high dose of ICS, respectively.
Sixty-two (82.7%) asthmatic children were using a spacer and 46 (61.3%)
children were washing the mouth after administration of ICS. In the
present study, the overall carriage rate of potential pathogens was 36%
for asthmatic children and 20% for controls (OR=2.25, 95% CI=0.75-6.67,
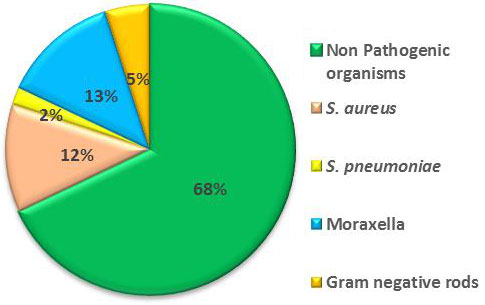
P=0.13). Fig. 1 illustrates an overview of the
carriage rate of pathogenic organism. No significant age-wise (1-5 years
versus 6-15 years) differences have been observed in the
isolation rates of the pathogens in the present study. We did not find
any association between the level of control of asthma and carriage
rates.
TABLE I Baseline Characteristics of Children with Asthma and Non-Asthmatic Controls
|
Social Characteristics |
Cases (n=75) |
Control (n=25) |
|
Age category |
|
12-60 mo |
21 (28) |
7 (28) |
|
61-180 mo |
54 (72) |
18 (72) |
|
Mean (SD) age, mo |
89.9 (36.98) |
84.6 ( 35.47) |
|
Male gender |
51 (68) |
14 (56) |
|
Rural residence |
42 (56) |
15 (60) |
|
Use of biomass for cooking* |
33 (44) |
5 (20) |
|
Overcrowding |
23 (30) |
9 (36) |
|
Joint family |
51 (68) |
14 (56) |
|
Smoker father* |
38 (50.7) |
7 (28) |
|
Immunization status |
|
Not immunized |
39 (52) |
17 (68) |
|
Completely immunized |
19 (25.3) |
5 (20) |
|
Partially immunized |
16 (21.3) |
3 (12) |
|
Status unknown |
1 (1.3) |
0 |
|
*P<0.05; All values in n (%) except *mean (SD). |
 |
|
Fig. 1 Bacterial isolate from
nasopharynx in children (aged 1- 15 years) with (n=75) and
without asthma (n=25)
|
The pathogenic organisms were isolated in 30.3%,
33.3% and 66.7% of asthmatic children taking a low dose, medium dose and
high dose ICS, respectively. There was no statistically significant
association of using a high dose of inhaled corticosteroids with the
isolation of pathogenic organisms (OR=4.6, 95% CI=0.95-22.1, P=0.05).
Colonization with pathogenic organism was found in 44% of asthmatic
children who were taking inhaled corticosteroids for more than 1 year as
compared to 25% of asthmatic children who were on inhaled
corticosteroids for less than 1 year duration which was not
statistically significant (OR=2.3, 95% CI 087-6.46, P=0.08).
Table II depicts the isolation of pathogenic organisms among
asthmatic children stratified by various characteristics. Exposure to
biomass fuel was associated with higher colonization rates of pathogenic
organisms among asthmatic children (OR=3.4, 95% CI= 1.26-9.10, P=0.03).
TABLE II Nasopharyngeal Isolation of Pathogenic Organism Among Children with Asthma
|
Pathogenic organisms |
|
|
Present, n (%) |
OR (95% CI) |
|
Dose of ICS |
|
|
|
Low dose |
10(30.3) |
|
|
Moderate dose |
11(33.3) |
1.1 (0.4-3.24) |
|
High dose |
6(66.7) |
4.6 (0.95-22.10) |
|
Level of control |
|
|
|
Well controlled |
11(27.5) |
|
|
Moderately controlled |
10(43.5) |
2.0 (0.69-5.90) |
|
Uncontrolled |
6(50) |
2.6 (0.69-9.94) |
|
Mouth wash after ICS use |
13(28.3) |
3.1 (0.89-6.25) |
|
Use of spacer |
24(38.7) |
1.1 (0.07-2.12) |
|
*Child in exacerbation |
12(57.1) |
5.7 (0.10-0.82) |
|
*P=0.01. |
Discussion
The present study showed the lack of association
between the use of ICS and nasopharyngeal colonization by pathogenic
bacteria in asthmatics. Similar findings were also reported by another
study conducted in China in 2013 [7]. They found no significant
differences in bacterial isolation rates among controls and children
with asthma treated with ICS after 3, 6 and 12 months. Although in our
study, we did not do a longitudinal follow up. We could not find a
statistically significant association between the different doses of ICS
and carriage rate of the pathogenic organisms. Our results were also in
concordance with another similar study from Turkey [8].
We observed that children receiving higher doses of
ICS were more likely to be carriers of potentially pathogenic bacteria
than those receiving low and medium doses, although there was no
statistical effect on the carriage of pathogenic bacteria tested. This
could be because the number of children receiving high dose ICS was
small. Children in exacerbation had increased colonization with the
pathogenic organism as compared to children without exacerbations.
Although, evidence linking acute asthma exacerbations to bacterial
infections are limited. However, respiratory viruses may facilitate the
emergence of bacterial infections by impairing the anti-bacterial
defenses by human alveolar macrophages [9]. But, testing for viral
pathogens in nasopharyngeal swab was not done in the present study.
Usage of biomass fuel is a potential risk factor for colonization of
pathogenic organisms in asthmatic children.
There were some limitations in our study. We did not
follow up cases of asthma taking ICS for change in colonization
patterns. Skim milk, tryptone, glucose, and glycerin (STGG) medium was
not used for transport of nasopharyngeal swab, hence there was low
isolation of S. pneumoniae. Majority of the cases were taking one
pharmacological preparation of ICS, hence we could not assess the effect
of different types of ICS on nasopharyngeal colonization.
We conclude that ICS do not increase the colonization
of potentially pathogenic organisms and high doses of ICS are safe in
the treatment of asthma. We should avoid biomass fuels for cooking in
households as it increases the risk of colonization.
Contributors: GN: enrolled the patients,
collected the data, performed data analysis, drafted the initial
manuscript and approved the final manuscript as submitted; SA: conceived
the idea of this the study, supervised data collection, helped in data
analysis. reviewed it critically and approved the final manuscript as
submitted; SG: supervised data collection, and approved the final
manuscript as submitted; JA: supervised microbiological testing and
approved the final manuscript as submitted.
Funding: None; Competing Interests:
None stated
|
What This Study Adds?
• Inhalational corticosteroids do not appear
to increase the risk of nasopharyngeal colonization of potential
pathogenic organisms in children with asthma.
|
References
1. Dubus JC, Marguet C, Deschildre A, Mely L, Le Roux
P, Brouard J, et al. Local side effects of inhaled
corticosteroids in asthmatic children: influence of the drug, dose, age,
and device. J Allergy Clin Immunol. 2001;56:944-8.
2. Garcia-Rodriguez J, Fresnadillo M. Dynamics of NP
colonization by potential respiratory pathogens. J Antimicrob
Chemother. 2002;50:59-73.
3. Fukushima C, Matsuse h, Tomari S, Obase Y,
Miyazaki Y, Shimoda T, et al. Oral candidiasis is associated with
inhaled corticosteroid use: Comparison of fluticasone and beclomethasone.
Ann Allergy Asthma Immunol. 2003;90:646-51.
4. Arocha-Sandoval F, Parra-Quevedo K. Oropharyngeal
bacteria in asthmatic patients in the city of Maracaibo, Venezuela.
Invest. Clin. 2002;43:145-55.
5. Global Strategy for Asthma Management and
Prevention. Global Initiative for Asthma (GINA), National Heart, Lung
and Blood Institute, US Department of Health and Human Services:
National Institute of Health (NIH); 2015.
6. Collee JG, Marmion BP, Fraser AG, Simmons A.
Mackie & Mc Cartney Practical Medical Microbiology, 14th ed. India;
Elsevier; 2006.
7. Lin H, Sun Y, Lin RJ, Xv J, Li N. Influence of
inhaled corticosteroids on distribution of throat flora in children with
bronchial asthma. Chin J Otorhinolaryngol Head Neck Surg. 2010;45:656-9.
8. Talay F, Karabay O, Yilmaz F, Kocoglu E. Effect of
inhaled budesonide on oropharyngeal, Gram-negative bacilli colo-nization
in asthma patients. Respirology. 2007;12:76-80.
9. Oliver BG, Lim S, Wark P, Laza-Stanca V, King N,
Black JL, et al. Rhinovirus exposure impairs immune responses to
bacterial products in human alveolar
macro-phages. Thorax. 2008;63:519-25.
|
|
|
 |
|

