|
|
|
Indian Pediatr 2018;55: 993- 994 |
 |
Neuroschistosomiasis: An Unusual
Intracranial Space Occupying Lesion
|
|
Nevitha Athikari Manamal 1,
Tanu Singhal2,
Abhaya Kumar3,
Darshana Sanghvi4
and Jayanti Mani5
From Departments of 1Laboratory Medicine,
2Pediatrics, 3Neurosurgery, 4Radiology
and 5Neurology, Kokilaben Dhirubhai Ambani Hospital and
Medical Research Institute, Mumbai, India.
Correspondence to: Dr Tanu Singhal, Department of
Paediatrics, Kokilaben Dhirubhai Ambani Hospital and Medical Research
Institute, Mumbai 400 053, India.
Email: tanusinghal@yahoo.com
Received: October 22, 2017;
Initial Review: February 15, 2018;
Accepted: May 24, 2018.
|
Background: Neuroschistosomiasis
is an uncommonly reported disease. Case characteristics: An
adolescent Indian boy residing in Kenya presented with headache, visual
symptoms and seizures, with MRI showing space-occupying lesions in the
occipital lobe and cerebellum. Observation: Brain biopsy was
diagnostic of neuro-schistosomiasis; complete recovery was seen with
praziquantel and corticosteroid therapy. Message: This case
highlights the importance of considering epidemiology in differential
diagnosis and establishing definitive diagnosis even if it is by
invasive methods.
Keywords: Headache, Raised intracranial pressure, Schistosoma
hemotobium, Seizures.
|
|
S
chistosomiasis; although widely prevalent
worldwide [1,2], is rarely reported from India due to the absence of the
specific intermediate snail hosts [3]. Acute neuroschistosomiasis,
usually due to S. japonicum, presents as acute
meningo-cencephalitis, whereas, chronic neuroschistosomiasis results
from ectopic migration of the eggs from the portal mesenteric or pelvic
systems to the CNS leading to a granulomatous reaction to the eggs. [4].
These can present as Pseudotumoral encephalic schistosomiasis due to
S. japonicum, or Spinal cord schistosomiasis in S. mansoni/ S.
hematobium [4]. We describe here a case of neuroschistosomiasis in a
non-resident Indian adolescent living in Kenya.
Case Report
This 17-year-old boy of Indian origin residing in
Nairobi, Kenya had been experiencing headaches, light headedness and
episodes of blurred vision for two months before admission. He also
reported episodes of flickering balls of light in front of his eyes that
came on randomly multiple times a day and left behind a dull occipital
headache. There was no fever, weight loss or cough. Magnetic resonance
imaging (MRI) done in Kenya showed space occupying lesions in the right
occipital parenchyma and left cerebellum. He presented to our hospital
with a tonic clonic seizure. The physical examination was normal.
Antiepileptic therapy with levetiracetam was initiated.
Radiologic differentials of lymphoma, autoimmune
vasculitis and tuberculosis were considered. Initial investigations
revealed a normal complete blood count, ESR and CRP, and negative ANA
and ANCA. CSF study including a TB PCR was negative. A contrast enhanced
CT scan of the chest and abdomen done to look for extra CNS TB was
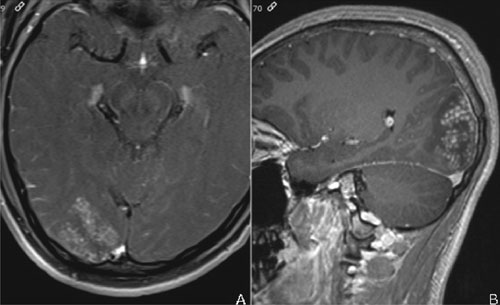
normal. Repeat MRI showed mild interval progression (Fig. 1).
Again seen were focal mass- like lesions in the right occipital lobe and
left cerebellum with prolonged T1- and T2-signal and perilesional
vasogenic edema. The lesions had ill-defined and irregular margins with
heterogenous enhancement. Arborised linear enhancement with interspersed
enhancing punctate nodules was seen. Punctate nodules formed the larger
conglomerate masses. As the diagnosis could not be established through
indirect and surrogate tests, an occipital craniotomy was done and brain
biopsy taken.
 |
|
Fig. 1 Axial (a) and sagittal (b) T1
weighted contrast MRI shows conglomerate nodular and linear
enhancing nodules with arborization.
|
On histopathology, the brain parenchyma showed
granulomatous inflammation with numerous well-formed granulomas composed
of epithelioid cells, multinucleate giant cells and central necrosis.
The periphery showed lymphoplasmacytic infiltrate along with eosinophils.
The center of many granulomas showed one or two non-operculated
refractile eggs/ova of trematode with terminal spine (Web Fig.
1). Few ova show ruptured and distorted morphology engulfed by giant
cells. No vasculitis or viable larvae of parasite were seen. Thus a
tentative diagnosis of schistosomiasis was made. The ova morphology with
a terminal spine resembled S. hematobium. The images were sent to
the Department of Parasitic Diseases at Centers for Disease Control,
Atlanta, USA where the diagnosis was confirmed.
On enquiry, the patient who was an avid swimmer gave
a history of frequent swimming in natural fresh water lakes in Kenya.
There was no history of urinary or intestinal symptoms, and his urine
and stool analysis were negative for parasitic eggs. His serum was
positive for schistomiasis by hemagglutination (2560, reference range
<160), ELISA (6.8, reference range <0.8 negative, 0.8-1.2 equivocal and
>1.2 positive) as well as Western Blot (positive for the p30-32 / p20-24
bands)
The patient was started on praziquantel at a dose of
40 mg/kg/day for 3 days along with dexamethasone 4 mg thrice daily which
was subsequently tapered over 1 month. His clinical symptoms resolved
completely and a repeat MRI after 2 weeks showed remarkable regression
of the CNS lesions. The boy was asymptomatic at the six-month follow-up,
and a repeat MRI was normal.
Discussion
Neuroschistosomiasis is commoner than perceived,
diagnosed or reported [4-6]. An autopsy study from Africa reported that
half the patients with urinary schistosomiasis had brain lesions [7].
Another pathological study in Africa found scattered ova of S.
haematobium or S. mansoni in the brain at autopsy in around
25% of 150 unselected cadavers [5]. Only a handful of cases of
indigenous urinary schistosomiasis have been reported from India, but
none of neuroschistosomiasis [3].
In the index case, commoner differentials like
tuberculosis, lymphoma, and autoimmune etiology were considered, but
biopsy revealed the rare etiology. Even in most cases reported from
endemic areas, the diagnosis is usually made inadvertently following
brain biopsy and lesion excision. This is because the clinical symptoms
of the disease are non-specific and mimicked by other infectious and
non-infectious illnesses, including tumors. Some investigators have
described arborised linear enhancement circled by multiple enhancing
punctuate nodules as more characteristic for schistosomiasis [8]. CSF
findings are also non-specific. Recent studies have reported on the
utility of specific schistosoma serology and PCR in CSF for diagnosis
[9]. In some cases, the speciation was done by PCR analysis of brain
tissue [5].
Treatment of chronic neuroschistosomiasis is not well
standardized but good results with combination therapy with praziquantel
and steroids [4] are reported. Current consensus is to reserve surgery
only for non-responding lesions, severely elevated intracranial pressure
and intractable epilepsy [10]. This case highlights the need to consider
epidemiology in clinical differential diagnosis of any illness as well
as the importance of establishing a definitive diagnosis even if it is
by highly invasive methods.
Acknowledgements: CDC (Centre for Disease
Control) DPD MDPDx team for confirmation of diagnosis and Dr Francois
Cornu from Eurofins, Biomnis, France.
Contributors: NAM: histopathologic diagnosis and
drafted the manuscript; TS: treatment and finalized the manuscript; AK:
performed the craniotomy and lesion excision and contributed to writing
the manuscript; DS: reported on the MRI and wrote parts of the
manuscript; JM: involved in clinical care of the patient and contributed
to the manuscript.
Funding: None; Competing interest: None
stated.
References
1. Gryseels B, Polman K, Clerinx J, Kestens L. Human
schistosomiasis. Lancet. 2006; 368: 1106-18.
2. WHO fact sheet updated January 2017. Available
from:
http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis.
Accessed June 12, 2017.
3. Kali A. Schistosome infections: An Indian
perspective. J Clin Diagn Res. 2015;9:DE01-4.
4. Ferrari TC, Moreira PR. Neuroschistosomiasis:
Clinical symptoms and pathogenesis. Lancet Neurol. 2011;10: 853-64.
5. Imai K, Koibuchi T, Kumagai T, Maeda T, Osada Y,
Ohta N, et al. Cerebral schistosomiasis due to Schistosoma
haematobium confirmed by PCR analysis of brain specimen. J Clin
Microbiol. 2011;49:3703-6.
6. Pollner JH, Schwartz A, Kobrine A, Parenti
DM. Cerebral schistosomiasis caused by Schistosoma haematobium: Case
Report. Clin Infect Dis.1994;18:354-7.
7. Alves W. The distribution of schistosoma eggs in
human tissues. Bull World Health Organ. 1958;18:1092-97.
8. Liu H, Lim CC, Feng X, Yao Z, Chen Y, Sun H, et
al. MRI in cerebral schistosomiasis: Characteristic nodular enhance-ment
in 33 patients. Am J Roentgenol. 2008;191:582-8.
9. Härter G, Frickmann H, Zenk S, Wichmann D, Ammann
B, Kern P, et al. Diagnosis of neuroschistosomiasis by antibody
specificity index and semi-quantitative real-time PCR from cerebrospinal
fluid and serum. J Med Microbiol. 2014;63:309-12.
10. Zhu F, Huang X, Wu M, Jin WX, Xie K. Diagnosis and treatment of
cerebral schistosomiasis: A report of 166 cases. Zhongguo Xue Xi Chong
Bing Fang Zhi Za Zhi. 2014;26: 695-6.
|
|
|
 |
|

