|
|
|
Indian Pediatr 2018;55:989-992 |
 |
Meta-analysis
Evaluating Efficacy and Safety of Levetiracetam for the
Management of Seizures in Children
|
|
Source Citation: Zhang L, Wang C, Li
W. A meta-analysis of randomized controlled trials on levetiracetam in
the treatment of pediatric patients with epilepsy. Neuropsychiatr Dis
Treat. 2018;14:769-79.
Section Editor: Abhijeet Saha
|
|
Summary
This meta-analysis was conducted to evaluate clinical
efficacy, safety and tolerability of levetiracetam as mono- or
adjunct-therapy in the treatment of children and adolescents with
epilepsy. A total of 1,013 patients were included from 13 randomized
controlled trials (RCTs). Levetiracetam had a comparable seizure-free
rate (RR 1.16, 95% CI 1.03, 1.31; P=0.30) compared to other
anticonvulsants (oxcarbazepine, valproate, sulthiame, carbamazepine) or
placebo. Seizure-frequency reduction of (>50% from baseline)
levetiracetam was equivalent (RR 1.08, 95% CI 1.01 to 1.16; P=0.35)
to other antiepileptic drugs (AEDs) with a comparable side effect
profile (RR 0.90, 95% CI 0.77, 1.06). The authors concluded that
levetiracetam had comparable efficacy, tolerability and adverse effect
profile compared to others AEDs, and advocated more well-designed trials
to justify widespread use of levetiracetam.
Commentaries
Evidence-based Medicine Viewpoint
Relevance: In recent years, levetiracetam has
gained popularity for treating seizure disorders in adults and children.
The National Institute for Health and Clinical Excellence (NICE)
guidelines in the United Kingdom recommended it as an add-on medication
for certain types of seizures such as partial seizures, myoclonic
seizures, and a limited number of other specific causes of childhood
seizures [1]. This is probably because the mechanism of anti-convulsant
action of levetiracetam is different from other medications. Its other
pharmacological properties, including near-complete absorption after
oral intake, minimal metabolism, and absence of drug interactions make
it attractive for clinical use. A recently published Cochrane review [2]
undertook a network meta-analysis to examine the comparative therapeutic
efficacy of ten AEDs. Although the review was not targeted to children
alone, it concluded that medications like phenobarbitone or phenytoin
had greater efficacy than newer agents, but these were also associated
with the highest risk of non-compliance compared to lamotrigine or
levetiracetam. Overall, the network meta-analysis suggested that
valproate is the drug of choice for generalized tonic-clonic seizures,
while lamotrigine or levetiracetam are other appropriate options.
Similarly, in partial seizures, carbamazepine and lamotrigine are
appropriate as initial therapy; although, levetiracetam could be an
option. Another systematic review [3] evaluating levetiracetam
monotherapy in children included 32 studies with various study designs,
but failed to find convincing evidence to support levetiracetam in
preference to other medications. A fairly recent systematic review
explored the safety profile of levetiracetam in children [4], and
identified a higher (but statistically insignificant) prevalence of
behavioral problems as well as drowsiness. Another systematic review in
adults and children also confirmed that the adverse event profile of
levetiracetam affected compliance to treatment [5]. Against this
background, this new systematic review focusing on the efficacy and
safety of levetiracetam in children has been published [6].
Critical appraisal: Table I
summarizes critical appraisal of the systematic review using one of
several tools available for the purpose [7]. However, several additional
issues emerged. It appears that the authors were more focused on the
meta-analysis component, rather than the systematic review of
literature. This is also reflected in the title’s emphasis on
meta-analysis, instead of the review itself. It should be clarified that
meta-analysis is only one component of systematic reviews, and
represents a statistical tool for pooling the results across included
studies to obtain a summary estimate of effect. Therefore, if the
systematic review is not conducted properly, the meta-analysis can be
flawed.
|
TABLE I Critical Appraisal of the
Systematic Review
|
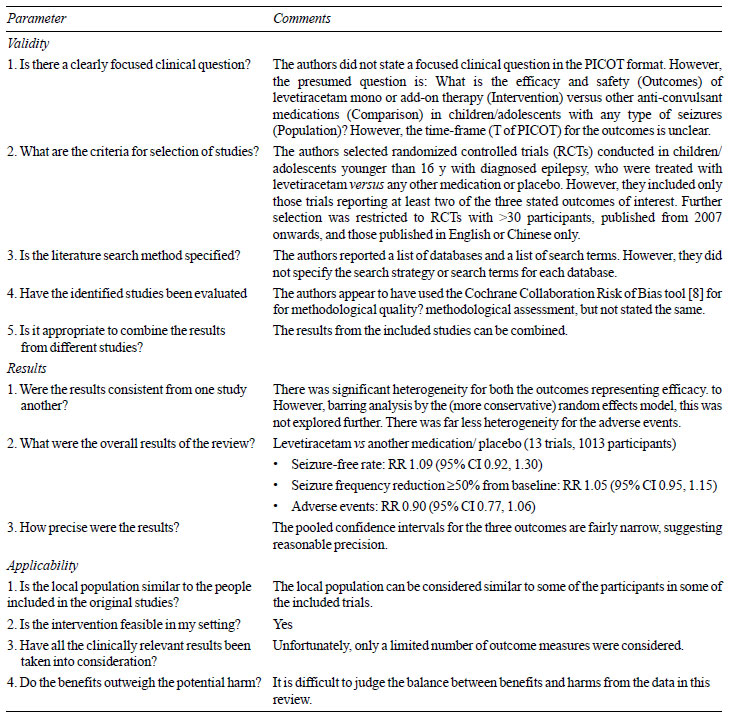 |
In this review [6], the authors applied several
restrictions to their literature search that have not been properly
justified. For example, trials were included only if they reported two
specific outcome measures of therapeutic efficacy viz
seizure-free rate and >50% frequency reduction from baseline.
This restriction limited the scope of considering trials with other
outcome measures of efficacy, such as seizure-free period, time to
seizure recurrence, time to withdrawal of medication, and
³75% frequency
reduction from baseline. Similarly, adverse events were represented in a
single outcome without emphasizing on serious events, and those leading
to therapy discontinuation. Thus, coupled with a single outcome measure
on adverse events, there were only three outcome measures in this
review. Further, the authors included only those RCTs reporting at least
two of these three outcomes. This reflects bias.
Although the review was focused on the pediatric age
group, only trials including children younger than 16 years of age were
included, and no justification for excluding children between 16 to 18
years was provided. Another serious restriction was the exclusion of
trials with less than 30 participants. These arbitrary restrictions
resulted in the exclusion of a trial by Rosenow, et al. [9]
comparing levetiracetam versus lamotrigine. The fact that this
trial included 33 children upto 17 years of age makes one wonder whether
arbitrary restrictions in this review [6] were designed to exclude this
trial [9]. Unfortunately, the authors did not present a ‘Table of
excluded studies’ for readers to judge whether any other eligible trials
were unfairly excluded. The ‘Table of Included studies’ [6] lacks
information on whether levetiracetam was used as mono- or polytherapy,
and whether it was the initial treatment or add-on treatment. Similarly,
the duration of therapy was also not described. These are important to
judge the clinical utility of levetiracetam.
Conference abstracts were completely excluded from
the review without providing a justification. Similarly, the language
restriction in the review without specifying reasons is also arbitrary;
although, this could be related to resource constraints and the focus on
applicability in the local population.
On the other hand, the authors decided not to
restrict inclusion of RCTs based on duration of treatment and/or
follow-up. This permitted the inclusion of three trials with very short
follow-up periods ranging from 5 days to 12 weeks. It is interesting
that all three were placebo-controlled, and together showed
statistically significant benefit with levetiracetam.
The authors did not explore heterogeneity observed in
the meta-analyses. It is essential to understand whether the efficacy of
levetiracetam differed on the basis of duration of treatment, baseline
clinical diagnosis, compliance to therapy, mono- or polytherapy, initial
or add-on agent, etc. The table showing quality assessment of the
included RCTs reported ‘very serious limitations’ for all three outcomes
despite the absence of any serious inconsistency, indirectness or
imprecision. However, no explanation was provided for this. Although
publication bias was assessed, the results were not presented.
The overall presentation of the systematic review [6]
has considerable room for improvement. For example, the text stated that
levetiracetam was superior to placebo for both the outcome measures
reflecting therapeutic efficacy. However, the two forest plots reflect
the exact opposite result, showing that placebo was superior to
levetiracetam. The Discussion section is heavily loaded with a
repetition of the results, without alluding to the existing systematic
reviews on the topic or the additional value of this review. On a
lighter note, data extraction was done in an ‘electric’ rather than
‘electronic’ format – a minor point that probably escaped the attention
of the editorial process.
Usually, events in RCTs are counted in terms of the
unfavorable outcome (i.e., treatment failure), rather than
favorable outcome (i.e., treatment success). Thus, in this review
[6], failure of seizure control and failure to achieve >50%
reduction in seizure frequency would be the conventional expression of
the outcome measures. Reversing the convention affects calculation of
relative risks as shown in Table II. Further, the dramatic
efficacy compared to placebo was considerably blunted.
Table II Comparison of Levetiracetam Efficacy by Expression of Outcome Measures
(Risk Ratio, Random Effects Model)
|
Review by Zhang [6]
|
Re-analysis of data
|
Review by Zhang [6]
|
Re-analysis of data
|
|
Event: Seizure-free
|
Event: Absence of |
Event: Seizure-free reduction |
Event: Absence of seizure-free
|
|
rate |
seizure-free state |
³50% from baseline
|
reduction ³50% from baseline |
|
vs Oxcarbazepine |
1.09 (0.95, 1.25) |
0.93 (0.72, 1.20) |
1.00 (0.93, 1.09) |
1.06 (0.69, 1.64) |
|
vs Placebo |
4.25 (1.92, 9.45) |
0.77 (0.61, 0.98) |
1.79 (1.26, 2.53) |
0.73 (0.62, 0.86) |
|
vs Sulthiame |
0.89 (0.70, 1.14) |
2.10 (0.43, 10.26) |
0.89 (0.70, 1.14) |
2.10 (0.43, 10.26) |
|
vs Valproate |
1.11 (0.88, 1.40) |
0.94 (0.80, 1.11) |
1.08 (0.93, 1.25) |
0.60 (0.25, 1.44) |
|
vs Carbamazepine |
0.64 (0.36, 1.16) |
1.49 (0.87, 2.54) |
0.76 (0.50, 1.15) |
1.65 (0.77, 3.53) |
|
Pooled estimate |
1.09 (0.92, 1.30) |
0.87 (0.77, 1.00) |
1.05 (0.95, 1.15) |
0.83 (0.67, 1.02) |
Extendibility: The clinical problem, type of
patients, therapeutic options and choice of medication administered, are
all extendible to our settings. Therefore, had this review been free
from the bias(es) highlighted, the results could have been considered
for application.
Conclusion: This systematic review [6] has
several methodological limitations that limit the confidence in the
reported results that levetiracetam has comparable efficacy and safety
with respect to other AEDs in children.
Funding: None; Competing interests: None
stated.
Joseph L Mathew
Department of Pediatrics,
PGIMER, Chandigarh, India.
Email:
[email protected]
References
1. National Institute for Health and Clinical
Excellence. The epilepsies: the diagnosis and management of the
epilepsies in adults and children in primary and secondary care 2012.
Available from: guidance.nice.org.uk/cg137. Accessed October 15,
2018.
2. Nevitt SJ, Sudell M, Weston J, Tudur Smith C,
Marson AG. Antiepileptic drug monotherapy for epilepsy: a network
meta-analysis of individual participant data. Cochrane Database Syst
Rev. 2017;6:CD011412.
3. Weijenberg A, Brouwer OF, Callenbach PM.
Levetiracetam monotherapy in children with epilepsy: A systematic
review. CNS Drugs 2015;29:371-82.
4. Egunsola O, Choonara I, Sammons HM. Safety of
levetiracetam in paediatrics: A systematic review. PLoS One.
2016;11:e0149686.
5. Verrotti A, Prezioso G, Di Sabatino F, Franco V,
Chiarelli F, Zaccara G. The adverse event profile of levetiracetam: A
meta-analysis on children and adults. Seizure. 2015;31: 49-55.
6. Zhang L, Wang C, Li W. A meta-analysis of
randomized controlled trials on levetiracetam in the treatment of
pediatric patients with epilepsy. Neuropsychiatr Dis Treat.
2018;14:769-79.
7. Abalos E, Carroli G, Mackey ME, Bergel E. Critical
appraisal of systematic reviews Available from: http://apps.who.int/rhl/Critical%20appraisal%20of%20
systematic%20reviews.pdf. Accessed December 14, 2016.
8. Cochrane Risk of Bias Tool (modified) for Quality
Assessment of Randomized Controlled Trials. Available from:
http://www.tc.umn.edu/~msrg/caseCATdoc/rct.crit. pdf. Accessed
November 20, 2017.
9. Rosenow F, Schade-Brittinger C, Burchardi N, Bauer
S, Klein KM, Weber Y, et al. The La Li Mo Trial: lamotrigine
compared with levetiracetam in the initial 26 weeks of monotherapy for
focal and generalized epilepsy- an open-label, prospective, randomised
controlled multicenter study. J Neurol Neurosurg Psychiatry.
2012;83:1093-8.
Pediatric Neurologist’s Viewpoint
Levetiracetam (LEV) has been the drug most widely
used off-label in pediatric population for past decade and half.
Initially FDA-approved as an adjunctive anti-epileptic drug (AED) for
adults with partial epilepsy, it was later approved for children with
partial-onset seizures (above 4 years of age), myoclonic seizures (6
years and older) and generalized tonic-clonic seizures (12 years and
older) [1]. In 2012, it was approved for usage in infants from 1 month
of age. It was welcomed with open arms by Neurologists caring for
infants and children, with hopes of efficacious seizure control with
novel mechanism of action and minimal adverse effects. The meta-analysis
by Zhang, et al. [2] brings out many pertinent issues to light –
good and bad – with Levetiracetam usage in children. The vast number of
studies/publications excluded (over 1000) in the meta-analysis
highlights its widespread use. On the flip side, only13 eligible studies
(8 from China) also points to lack of good quality evidence on its
efficacy and adverse events. Although LEV was more effective than
placebo, and equally effective as other AEDs (Valproate, Carbamazepine,
Sulthiame, Oxcarbazepine), in reducing seizures by >50%, it has not
shown superiority over other AEDs or placebo in terms of 100%
seizure-free rate. In the subgroups, LEV was slightly better in children
with Rolandic epilepsy than other partial epilepsies, but this was not
statistically significant. The adverse events were also similar to other
AEDs. There are lot of limitations in interpreting this data: large
heterogeneity of studies with differing sample sizes, variable intervals
for efficacy estimation, heterogeneity of epilepsies studied etc.
With the available studies, it is difficult to make any meaningful
conclusions about the efficacy of LEV from this review. As epilepsies in
infants and children are diverse, it is hoped that homogenous
populations (e.g., epileptic spasms, benign focal epilepsies,
primary generalized epilepsies, infantile-onset epilepsies of unknown
etiology, focal epilepsies of structural etiology) are studied in
future, with clinically meaningful intervals for end-point estimation.
For now, it’s a long way before LEV can be accorded prime position in
pediatric AED armamentarium based on current evidence.
Funding: None; Competing interests: None
stated.
Ramesh Konanki
Consultant Pediatric Neurologist
Rainbow Hospital for Women and Children,
Hyderabad, India.
Email: rameshkonanki@gmail.com
References
1. Weijenberg A, Brouwer OF, Callenbach PM.
Levetiracetam monotherapy in children with epilepsy: a systematic
review. CNS Drugs. 2015;29 :371–82.
2. Zhang L, Wang C, Li W. A meta-analysis of randomized controlled
trials on levetiracetam in the treatment of pediatric patients with
epilepsy. Neuropsychiatr Dis Treat. 2018;14:769-79.
|
|
|
 |
|

