|
|
|
Indian Pediatr 2018;55: 951-956 |
 |
Supplementation with Three Different Daily
Doses of Vitamin D 3
in Healthy Pre-pubertal School Girls: A Cluster Randomized Trial
|
|
Raman Kumar Marwaha 1,
A Mithal2, Neetu
Bhari3, G
Sethuraman3,
Sushil Gupta4,
Manoj Shukla4,
Archana Narang 5,
Aditi Chadda5,
Nandita Gupta6, V
Sreenivas7 and MA
Ganie6
From 1International Life Sciences
Institute (India); 2Medanta Hospital Gurgaon, Haryana;
Departments of 3Dermatology, 6Endocrinology and
7Biostatistics, AIIMS, New Delhi; 4SGPGI, Lucknow,
Uttar Pradesh; and 5Dr BR Sur Homeopathic Medical College,
New Delhi; India.
Correspondence to: Maj Gen Raman Kumar Marwaha,
Scientific Advisor (Projects), International Life Science Institute
(India).
[email protected]
Received: September 28, 2017;
Initial review: February 19, 2018;
Accepted: September 04, 2018.
Trial registration: Clinical Trial Registry of
India (CTRI): 2017/01/007681
|
|
Objective: To compare the adequacy and efficacy of different doses
of vitamin D3 in pre-pubertal girls.
Design: Cluster Randomized
controlled trial.
Setting: Public school in Delhi,
India, between August 2015 and February 2016.
Participants: 216 healthy
pre-pubertal girls, aged 6.1-11.8 years.
Intervention: Daily
supplementation with 600 IU (n=74), 1000 IU (n=67) or 2000
IU (n=75) of vitamin D3 under supervision for 6 months.
Outcome measures: Primary:
Rise in serum 25 hydroxy Vitamin D (25(OH)D); Secondary: Change
in bone formation and resorption markers.
Results: Following 6 months of
supplementation, the mean (SD) rise in serum 25(OH)D was maximum with
2000 IU (24.09 (8.28) ng/mL), followed by with 1000 IU (17.96 (6.55) ng/mL)
and 600 IU (15.48 (7.00) ng/mL). Serum 25(OH)D levels of
³20 ng/mL
were seen in 91% in 600 IU group , 97% in 1000 IU group and 100% in 2000
IU group. The overall mean (SD) rise in urinary calcium creatinine ratio
(0.05 (0.28) to 0.13 (0.12) mg/mg), and serum procollagen type I
N-terminal propeptide (538.9 (199.78) to 655.5 (218.24) ng/mL), and
reduction in serum carboxy-terminal telopeptide (0.745 (0.23) to 0.382
(0.23) ng/mL) was significant (P<0.01). The change in the above
parameters was comparable among the three groups after adjustment for
age.
Conclusion: Daily vitamin D
supplementation with 600 IU to 2000 IU for 6 months results in Vitamin D
sufficiency in >90% of pre-pubertal girls.
Keywords: Micronutrient
supplementation, Prevention, Vitamin D deficiency.
|
|
V itamin D deficiency is a
widely recognized public health problem world over, including India.
There are limited studies on vitamin D supplementation in Indian
children, more so regarding adequate dose of vitamin D3 supplementation
in pre-pubertal children [1,2]. Vitamin D deficiency causes secondary
hyperparathyroidism with negative consequences on bone mineral density
(BMD) resulting in increase in serum bone resorption markers. There are
not many studies exploring the impact of vitamin D3 supplementation on
serum bone markers in children. In view of the above, we conducted this
study to compare the efficacy of daily supplementation of 600 IU, 1000
IU and 2000 IU vitamin D3 in pre-pubertal girls; and to evaluate the
effect of vitamin D3 supplementation on serum bone formation and
resorption markers.
Methods
This was a cluster randomized controlled trial
performed between August 2015 and February 2016. The study protocol was
approved by Institute Ethical Committee. Apparently healthy pre-pubertal
school girls (age 6.1-11.8 y), who consented to participate were
evaluated from a private school (representing an upper socio-economic
strata) in Delhi, India with consent from school authorities,
parents/guardians and verbal assent from children. Girls who were either
unable to swallow the capsule or were receiving drugs affecting bone
mineral metabolism (e.g. calcium, vitamin D, glucocorticoids,
anti-tubercular or anti-epileptics), or those suffering from any
systemic illness were excluded from the study. Eligible and consenting
girls were enrolled and randomized into three groups to be supplemented
with daily 600 IU (group A), 1000 IU (group B) 2000 IU (group C) of
vitamin D3 (capsule form) under supervision for 6 months. There were 4
classes (2nd to 5th) undertaken for supplementation with each class
having 3 sections with approximately 40 students per section. Cluster
randomization was done within each class considering each section of the
class as a cluster. For each class, sections were allocated for
interventions using simple random sampling with the help of drawing one
chit from three. The randomly allocated interventions were neither
shared with class teachers nor the students within each class till the
end of the study. The concealment was carried out by removing the labels
from the bottles. The different doses of vitamin D3 were procured as
capsules of same shapes but different colours, to avoid mix up and
cross-contamination in the allocation arms. Investigators were aware
about the intervention allocation to sections; though, the people
involved in the laboratory analysis were blinded to the intervention
status. The vitamin D3 capsules were soft gelatine capsules (D rise, USV
Pharma Ltd.) manufactured and supplied every month with no overages
added.
Baseline height was recorded to the nearest 1 mm
using Holtain stadiometer without wearing shoes and weight was recorded
to the nearest 0.1 kg by using digital weighing machine. Body mass index
(BMI) was calculated as weight (in kg)/ height (m 2).
Blood samples were collected in the fasting state between 0800 Hrs to
0900 Hrs. Serum 25(OH)D was estimated by chemiluminescence (Diasorin,
Stillwater, MN, USA) and parathyroid hormone (PTH) was measured using
electro chemiluminiscence method (Roche Diagnostics). Calcium,
phosphates, alkaline phosphatase were estimated by auto analyzer (Roche
Diagnostics USA). Serum procollagen type I N-terminal propeptide (PINP)
and carboxy-terminal telopeptide (CTX) were measured by Elecsys 2010,
based on the principle of electro-chemiluminescence immunoassay. Urinary
samples were also collected for the spot calcium /creatinine ratio and
was performed using Cobas C III (Roche). Repeat collection of fasting
blood and urine samples was undertaken one day after the completion of
supple-mentation. Vitamin D deficiency was defined as per Lips criteria;
mild (10-20 ng/mL), moderate (5-10 ng/mL), and severe (<5 ng/mL) [3].
Secondary hyperparathyroidism was defined as serum PTH levels >65 pg/mL.
Daily supplementation for 6 days/week was done for a
period of 6 months, under supervision of teachers and investigating
staff at the study site. Required numbers of vitamin D capsules were
provided to the parents/guardians every month along with a record sheet
to be maintained by the parents for Sundays and planned holidays as per
school calendar. For unplanned holidays, parents were advised to collect
their requirement from school.
Sample size calculation was based on our earlier
study where 70% and 81% of children achieved serum 25(OH)D levels of
³20 ng/mL when
supplemented with 600 IU and 1000 IU of vitamin D, respectively for 6
months and 90% proportion was expected with 2000 IU [4].
In order to detect a significant difference among
the 3 groups in a 2-sided test with a 5%
a error and 80%
power, 74 patients per group were required. Considering 10% loss during
the follow-up period, a sample size of 82 per group was calculated.
Statistical analysis: The proportion of subjects
achieving the desirable levels of 20 ng/mL at the end of intervention
were compared among the three study groups using chi-square test.
Analysis of variance (ANOVA) was used to study the difference in the
mean of various parameters, among the three study groups. Multiple
linear regression analysis was carried out on change in biochemical and
hormonal parameters. A P value of <0.05 was considered
statistically significant. Analysis was performed using Stata 11.0
(College station Road, TX, USA).
Results
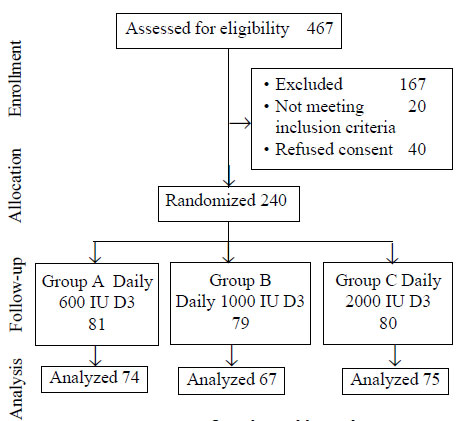
We approached 467 girls out of which 300 apparently
healthy girls who consented were evaluated. All 300 girls had 25 (OH) D
below 20 ng/mL. Out of these 300 girls, 240 were found eligible and
consented for study; 216 completed the study. Twenty-four were excluded
due to lack of proper follow-up, change of school, unavailability of
post-treatment laboratory reports, or missed taking supplementation for
more than 7 days continuously. Study flow is depicted in Fig.
1. Baseline hormonal and biochemical parameters and the changes
following 6 months of vitamin D3 supplementation are shown in
Table I and II, respectively.
 |
|
Fig. 1 Consort flow chart of the
trial.
|
TABLE I Baseline Hormonal and Biochemical Parameters in Pre-pubertal Girls
|
600 IU (n=74) |
1000 IU(n=67) |
2000 IU (n=75) |
Total(n=216) |
|
Serum 25(OH)D (ng/mL) |
10.13 (3.51) |
10.21 (3.71) |
9.8 (3.73) |
9.99 (3.64) |
|
Serum parathyroid hormone (pg/mL) |
43.27 (14.99) |
47.90 (23.69) |
57.19 (35.85) |
49.72 (27.22) |
|
Serum procollagen type-I N propeptide
|
557.44 (211.89) |
508.63 (166.52) |
560.43 (218.31) |
538.9 (199.78) |
|
(PINP) levels (mcg/L) |
|
|
|
|
|
Serum C-terminal telopeptide of type I collagen
|
0.856 (0.24) |
0.649 (0.18) |
0.683 (0.16) |
0.745 (0.23) |
|
(CTX) levels (mcg/L) |
|
|
|
|
|
Serum Calcium (mg/dL) |
9.43 (0.97) |
9.19 (0.46) |
9.59 (0.77) |
9.38 (0.77) |
|
Serum phosphate (mg/dL) |
5.22 (0.61) |
5.77 (5.01) |
5.34 (0.66) |
5.45 (3.08) |
|
Serum alkaline phosphatase (IU/L) |
256.33 (69.30) |
257.03 (61.72) |
276.04 (68.23) |
261.95 (66.93) |
|
Urinary calcium creatinine ratio (mg/mg) |
0.04 (0.46) |
0.03 (0.05) |
0.03 (0.03) |
0.05 (0.28) |
|
Values in mean (SD). |
TABLE II Change in Hormonal and Biochemical Parameters After Vitamin D3 Supplementation in Pre-pubertal Girls
|
Change from baseline |
600 IU (n=74) |
1000 IU(n=67) |
2000 IU (n=75) |
|
Serum 25(OH)D (ng/mL)*
|
14.93 (10.48, 18.67) |
18 (13.1, 21.5) |
22.21 (18.38, 28.69) |
|
Serum PTH (pg/mL)* |
6.26 (-1.29, 11.33) |
14.99 (6.51, 24.35) |
17.62 (10.1, 28.58) |
|
Serum PINP (mcg/L)*
|
54.29 (-59.24, 225.15) |
158.55 (40.99, 270.25) |
53.10 (-64.20, 216) |
|
Serum CTX (mcg/L)*
|
0.38 (0.10, 0.61) |
0.29 (0.18, 0.47)
|
0.41 (0.27, 0.62) |
|
Serum Calcium (mg/dL) |
0.5 (-0.19, 1.0)* |
0.6 (0.29, 1.0)* |
0.5 (-0.1, -0.8) |
|
Serum phosphate (mg/dL) |
0.14 (-0.39, -0.59) |
0.10 (-0.26, 0.73) |
0.24 (-0.04, 0.92)* |
|
Serum alkaline phosphatase (IU/L) |
-8 (-39, 32) |
-20 (-63, 33) |
-33 (-72,52) |
|
Urinary calcium creatinine ratio (mg/mg) |
0.07 (0.02, 0.16) |
0.05 (0.01, 0.20) |
0.02 (0.003, 0.08) |
|
Values represented as median (IQR); *P<0.05; PTH:
Parathyroid hormone; PNPP: Procollagen type-I N propeptide; CTX:
C-terminal telopeptide of type I collagen. |
All included girls had 25(OH)D levels below 20 ng/mL.
Mild, moderate, and severe deficiency was observed in 96 (44.4%), 113
(52.3%) and 7 (3.3%) children, respectively. Post-supplementation mean
(SD) serum 25(OH)D levels increased to 29.23 (8.00) ng/mL (P<0.01),
and a level of 20 ng/mL or more were seen in 67 (91%) girls in group A,
64 (97%) in group B and all (100%) in group C. The difference in the
rise of serum 25(OH)D levels between group A and C (7.74 ng/mL, P<0.01)
and between groups B & C (5.86 ng/mL, P<0.01) was significant.
The baseline serum PTH was 49.6 (27.2) pg/mL that decreased to 33.7
(14.5) pg/mL following 6 months of vitamin D3 supplementation (P<0.01).
Secondary hyperparathyroidism was seen in 32 (14.8%) children at
baseline, which reduced to 4.5% on follow-up (P<0.01). The mean
(SD) urinary calcium creatinine ratio increased from 0.05 (0.28) to 0.13
(0.12) mg/mg following 6 months of supplementation (P<0.01) that
was not different among the three groups after adjustment for age.
Following supplementation, serum PINP levels
increased significantly from 538.9 (199.78) to 655.5 (18.24) ng/mL (P<0.01)
and serum CTX decreased significantly from 0.745 (0.23) to 0.382 (0.23)
ng/mL (P<0.01); significant for intra-group comparison but not
intergroup comparison. No adverse effects were noted in any of the
participants during the study period.
Discussion
In the current study, a significant dose-dependent
increase in serum 25(OH)D with a significant reduction in mean PTH
levels was observed following 6 months of vitamin D3 supplementation.
Persistent secondary hyperparathy-roidism despite achieving serum 25
(OH) D ³20 ng/mL
following supplementation was noted in few subjects. Evaluation of bone
markers showed a marked increase in serum PINP and significant reduction
in CTX levels.
Major limitations of the study were (i)
inability to carry out 24-hour urinary calcium excretion, (ii)
absence of boys and adolescent girls in the study group, (iii)
lack of detailed dietary evaluation of calcium and vitamin D and (iv)
lack of intention to treat analysis. We chose daily dose of 600 IU as it
is recommended by Indian Academy of Pediatrics (IAP) and Institute of
Medicine (IOM), a higher dose of 1000 IU as per our earlier reported
prediction equation, and 2000 IU as per one recent study showing that
2098 IU of daily vitamin D supplementation is able to achieve serum
25(OH)D levels of ³20
ng/mL in 97.5% of children [4-7]. We did not include a placebo arm as
only vitamin D deficient children were included in the current study.
A dose-dependent increase in serum 25 (OH) D levels
has been reported in earlier studies evaluating the impact of vitamin D3
supplementation in different doses in children with vitamin D deficiency
[8-10]. The response to daily supplementation with 2000 IU of vitamin D3
in the current study was similar to that reported by Dong, et al.
[8] in American black boys (60 nmol/L) in contrast to Lewis, et al.
[10] where the increase was only 38 nmol/L. Similarly, the percentage of
vitamin D deficient Lebanese children who achieved vitamin D sufficiency
following 2000 IU/day of vitamin D3 supplementation for a year [9] was
similar to present study. The estimated intake of 2098 IU/day needed to
maintain serum 25(OH)D concen-tration at 20 ng/mL in 97.5% of US
children was in sharp contrast to 1000 IU/day required in the present
study to achieve sufficiency in 97% subjects [6,11].
The effect of vitamin D3 supplementation on bone
markers is less well studied. Few studies in children with vitamin D
deficiency or insufficiency have observed higher levels of plasma
osteocalcin, CTX and bone-specific alkaline phosphatase (BAP),
suggesting the role of vitamin D in maintenance of bone turn-over
[12-14]. Rajakumar, et al. [15] evaluated the effect of vitamin
D3 supplementation on serum bone markers in obese and non-obese children
aged 6-10-years with 400 IU of vitamin D3 daily for one month and noted
a significant increase in serum 25(OH)D with a decrease in serum
osteocalcin, BAP and urine n-telopeptide cross-links of type 1 collagen
(urine NTX) in both the groups. We observed a significant decrease in
serum CTX and PTH as has also been observed in a previous study [16].
However, the increase in serum PINP without significant decline in ALP
following vitamin D3 supplementation as noted in the present study was
possibly due to the normal growing phase in this age group. This is in
contrast to earlier studies in children and adults which observed
decrease in both formation and resorption markers [16-18]. The increase
in PINP levels, however, are consistent with the results of a study by
Ghazi, et al. [17] who observed an increase in osteocalcin and
alkaline phosphatase which are bone formation markers after monthly and
bimonthly vitamin D supplementation.
The urinary calcium to creatinine ratio shows a wide
variation ranging from 0.024 to 0.44 in various geographic areas,
including India [20-22]. The ratio in the present study significantly
increased post supple-mentation. The change in this ratio in children
following vitamin D3 supplementation has not been studied earlier.
Although, urinary calcium concentration is considered to monitor the
inadvertent vitamin D toxicity, levels may be affected by improperly
timed collections, missed urine voids, and daily variations in calcium
intake. Veith, et al. [23] had shown good correlation between
first-morning urine sample and 24-h urinary calcium excretion, though,
others have shown conflicting results [24]. Furthermore, it is highly
controversial whether isolated high-normal calcium excretion contributes
to stone disease or bone health [25]. Further studies are required to
confirm the significance of this finding.
Supplementation with all three daily doses of vitamin
D3 resulted in significant increase in the serum 25(OH)D levels. Higher
daily dose requirement of 1000 IU to achieve and maintain vitamin D
sufficiency in 97% of girls as against 600 IU/day as recommended by IAP
and IOM may be due to several confounding factors such as poor dietary
intake of calcium, limited sun exposure and lower serum baseline 25(OH)D
values [4,5]. It is therefore important to undertake well-planned
studies to ensure whether RDA of 600 IU recommended by IAP and IOM would
suffice to achieve and maintain serum 25(OH)D
³20 ng/mL in
prepubertal girls.
Acknowledgements: R Goswami, Professor,
Endocrinology, All India Institute of Medical Sciences, New Delhi, India
for performing serum calcium, phosphate, alkaline phosphatase and
urinary calcium creatinine ratio; and Ashish Kumar for his help in
collection of blood samples. We would also like to appreciate the help
rendered by the members of Society for Endocrine Health for Elderly,
Adolescence and Children (SEHEAC).
Contributors: RKM: conceptualized and designed
the study, manuscript writing; AM: conceptualized the study; NB: data
collection and manuscript writing; GS: data collection and manuscript
writing; SG: collection of blood samples and evaluation of bone markers;
MS: collection of blood samples and evaluation of bone markers;
AN,AC,NG: data collection; VS: statistical analysis; MAG: data
collection and analysis.
Funding: Endocrine and Diabetic foundation of
India, New Delhi, India. Vitamin D3 capsules were provided by USV
Pharmaceuticals.
Competing interest: AM is the founding president
of the Endocrine and Diabetic foundation of India.
|
What is Already Known?
• Recommended dietary allowance (RDA) for
vitamin D in children (beyond infancy) by Institute of Medicine
(US) and Indian Academy of Pediatrics (IAP) is 600 IU/day.
What This Study Adds?
• Supplementation with 600 IU/d results in
adequate 25(OH)D levels in 90% of pre-pubertal girls.
• Higher daily dose requirement of 1000 IU
was required to achieve and maintain vitamin D sufficiency in
97% of girls.
|
References
1. Balvers MG, Brouwer-Brolsma EM, Endenburg S, de
Groot LC, Kok FJ, Gunnewiek JK. Recommended intakes of vitamin D to
optimize health, associated circulating 25-hydroxyvitamin D
concentrations, and dosing regimens to treat deficiency: workshop report
and overview of current literature. J Nutr Sci. 2015;4:e23.
2. GR, Gupta A. Vitamin D deficiency in India:
Prevalence, causalities and interventions. Nutrients. 2014;6:729-75.
3. Lips P. Vitamin D deficiency and secondary hyper-parathyroidism
in the elderly: consequences for bone loss and fractures and therapeutic
implications. Endocr Rev 2001;22:477.
4. Khadgawat R, Marwaha RK, Garg MK, Ramot R, Oberoi
AK, Sreenivas V, et al. Impact of vitamin D fortified milk
supplementation on vitamin D status of healthy school children aged
10-14 years. Osteoporos Int. 2013;24: 2335-43.
5. Khadilkar A, Khadilkar V, Chinnappa J, Rathi N,
Khadgawat R, Balasubramanian R, et al. Prevention and Treatment
of Vitamin D and Calcium Deficiency in Children and Adolescents: Indian
Academy of Pediatrics (IAP) Guidelines. Indian Pediatr. 2017;54:567-73.
6. Aguirre Castaneda R, Nader N, Weaver A, Singh R,
Kumar S. Response to vitamin D3 supplementation in obese and non-obese
Caucasian adolescents. Horm Res Paediatr. 2012;78:226-31.
7. Narasinga Rao BS. Nutrient Requirements and
Recommended Dietary Allowances for Indians. A report of the Expert Group
of the Indian Council of Medical Research. NFI Bulletin. 2010;31:1-5.
8. Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris
RA, Keeton D, Huang Y, et al. A 16-week randomized clinical trial
of 2000 international units daily vitamin D3 supplementation in black
youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin
Endocrinol Metab. 2010;95:4584-91.
9. Al-Shaar L, Mneimneh R, Nabulsi, Maalouf J,
Fuleihan Gel-H. Vitamin D3 dose requirement to raise 25-hydroxyvitamin D
to desirable levels in adolescents: results from a randomized controlled
trial. J Bone Miner Res. 2014;29:944-51.
10. Lewis RD, Laing EM, Hill Gallant KM, Hall DB,
McCabe GP, Hausman DB, et al. A randomized trial of vitamin D
supplementation in children: dose-response effects on vitamin D
metabolites and calcium absorption. J Clin Endocrinol Metab.
2013;98:4816-25.
11. Rajakumar K, Moore CG, Yabes J, Olabopo F,
Haralam MA, Comer D, et al. Estimations of dietary vitamin D
requirements in black and white children. Pediatr Res. 2016;80:14-20.
12. Cheng S, Tylavsky F, Kröger H, Kärkkäinen M,
Lyytikäinen A, Koistinen A, et al. Association of low
25-hydroxyvitamin D concentrations with elevated parathyroid hormone
concentrations and low cortical bone density in early pubertal and
prepubertal Finnish girls. Am J Clin Nutr. 2003;78:485-92.
13. Foo LH, Zhang Q, Zhu K, Ma G, Hu X, Greenfield H,
et al. Low vitamin D status has an adverse influence on bone
mass, bone turnover, and muscle strength in Chinese adolescent girls. J
Nutr. 2009;139:1002-07.
14. Fares JE, Choucair M, Nabulsi M, Salamoun M,
Shahine CH, Fuleihan Gel-H. Effect of gender, puberty, and vitamin D
status on biochemical markers of bone remodedeling. Bone.
2003;33:242-47.
15. Rajakumar K, Fernstrom JD, Holick MF, Janosky JE,
Greenspan SL. Vitamin D status and response to Vitamin D (3) in obese
vs. non-obese African American children. Obesity (Silver Spring).
2008;16:90-95.
16. Kruger MC, Chan YM, Kuhn-Sherlock B, Lau LT, Lau
C, Chin YS, et al. Differential effects of calcium- and vitamin
D-fortified milk with FOS-inulin compared to regular milk, on bone
biomarkers in Chinese pre- and postmenopausal women. Eur J Nutr.
2016;55:1911-21.
17. Ghazi AA, Hosseinpanah F, Abdi H, Hedayati M,
Hasheminia M, Ghazi S, et al. Effect of different doses of oral
cholecalciferol on serum 1,25(OH)2D in vitamin D deficient school
children. Horm Metab Res. 2016;48: 394-98.
18. Penido MG, Diniz JS, Guimarães MM, Cardoso RB,
Souto MF, Penido MG. Urinary excretion of calcium, uric acid and citrate
in healthy children and adolescents. J Pediatr (Rio J). 2002;78:153-60.
19. Sönmez F, Akçanal B, Altincik A, Yenisey C.
Urinary calcium excretion in healthy Turkish children. Int Urol Nephrol.
2007;39:917-22.
20. So NP, Osorio AV, Simon SD, Alon US. Normal
urinary calcium/creatinine ratios in African-American and Caucasian
children. Pediatr Nephrol. 2001;16:133-39.
21. Sorkhi H, Haji Aahmadi M. Urinary calcium to
creatinine ratio in children. Indian J Pediatr. 2005;72:1055-56.
22. Rath B, Aggarwal MK, Mishra TK, Talukdar B,
Murthy NS, Kabi BC. Urinary calcium creatinine ratio and hypercalciuria.
Indian Pediatr. 1994;31:311-16.
23. Vieth R, Chan PC, MacFarlane GD. Efficacy and
safety of vitamin D3 intake exceeding the lowest observed adverse effect
level. Am J Clin Nutr. 2001;73:288-94.
24. Alconcher LF, Castro C, Quintana D, Abt N, Moran
L, Gonzalez L, et al. Urinary calcium excretion in healthy school
children. Pediatr Nephrol. 1997;11:186-8.
25. Riess C, Hess B, Binswanger U. Questionable significance of the
chemical analysis of a single 24-hour urine sample in recurrent calcium
oxalate nephrolithiasis. Klin Wochenschr. 1986;64:411-16 [German].
|
|
|
 |
|

