nflammatory Bowel disease (IBD) is a perplexing
disease characterized by chronic mucosal inflammation. It results from a
complex interplay of various factors including genetic and
environmental, and adaptive immunity of the host. Crohn’s disease (CD)
and Ulcerative colitis (UC) are the two broad phenotypes of IBD. CD is
characterized by its ability to involve any part of the gastrointestinal
tract in a discontinuous fashion. The inflammation associated with CD is
often transmural and granulomatous. UC on the other hand tends to
involve the rectum and the adjoining colonic mucosa to a variable
extent; albeit in a continuous fashion. The inflammation in UC is
usually superficial when compared with CD. The term indeterminate
colitis or IBD-U is used when the clinical and histopathological
features are unable to distinguish between CD and UC [1]. Early onset
IBD is important as researchers believe that it has a distinct phenotype
when compared with adult onset IBD. Moreover, the genetically
attributable risk is considered to be higher in early onset IBD, as
exposure to environmental factors is proportionately less.
Epidemiology
Multiple studies have shown that 25% of all IBD cases
have their onset in children less than 18 years of age [2]. However, the
incidence of the disease seems to be increasing internationally. A
systematic review of international trends in pediatric IBD revealed a
statistically significant increase in the period 1950-2009. The SPIRIT
registry from Spain collected data in 2100 pediatric patients with IBD
(1996-2009). It showed a collective increase in incidence of IBD from
0.97 to 2.8/100,000 inhabitants <18 years/year in the study period. The
median age at diagnosis was 12 years and the increase in CD cases was
more than UC cases, with males being majorly affected [3]. A similar
registry from Italy (1996-2003) showed a similar rise in the overall
incidence of IBD cases from 0.89 to 1.39/10 in children <18 years of
age. However, in this registry UC cases showed a greater increase than
CD cases [4]. The incidence of IBD in a prospective study (<16 years)
from UK was 5.2/100000 individuals/year. The proportion of CD was 60%,
while the proportion of UC was 28%. The mean age at diagnosis was 12
years. Studies from other European countries have shown incidence rates
of 0.6-6.8/100000 individuals/year for CD and 0.8-3.6 for UC. An
evaluation of North American studies revealed an incidence of 3-4/100000
individuals/year. Although studies and data are lacking from South
American, African and Asian nations, temporal trends are obvious from
the studies in the western hemisphere [5]. There is a male preponderance
in pediatric CD (1.5:1), while UC affects both sexes equally. CD is more
common in children as compared to UC (2.8:1) when compared with adult
data (0.85:1). CD in children presents more commonly as ileocolonic or
colonic disease. UC presents commonly (85-90%) as pancolitis [6].
Pediatric CD is predominantly an inflammatory disease; stricturing and
penetrating variants are rarely seen at presentation. UC, as mentioned
previously presents with a more severe phenotype which requires surgery
more often as compared to the adult phenotype [7].
Data from India is limited. The first case series on
CD was published from Southern India in 2005,detailing 10 children (5-15
years) with Crohn’s disease [8]. There was female preponderance (9 out
of 10), and interestingly, 50% of the children had received
antitubercular therapy prior to diagnosis. Another tertiary referral
center from Southern India reported 34 children with IBD (23 with CD and
11 with UC). These cases accounted for 7% of the total IBD load
presenting to that centre. The proportion of IBD was 0.03% of all
pediatric cases presenting to the outpatient department, and the median
delay in diagnosis was 15 months [9]. A recent questionnaire-based
survey from seven centers across India in 221 children and adolescents
with IBD showed that children with IBD in India have features similar to
adult-onset IBD. UC was present in 42% of these children while CD was
found in 55%; the rest were classified as indeterminate colitis. These
children shared similarities with adult-onset IBD in terms of
distribution of the disease. However, as in other reports on IBD in
children, growth failure and more severe forms of the disease were
commonly observed. The UC cases had complications like toxic megacolon
and bleeding in 12%, while 27% of CD cases had complications (fistulae,
strictures, perforation). Biological agents were used in less than 1% of
UC cases and in 12% of CD cases [10].
Genetics and environmental influence
Pediatric IBD has alerted researchers to the
possibility of genetic susceptibility playing a role in disease
pathogenesis. Epidemiological studies have highlighted a familial
association in 25-30% cases of pediatric IBD. The NOD2 gene for
CD and the MHC region on 6p for UC were two of the first genes to
be implicated in disease causation. With the availability of Genome wide
association scanning (GWAS) using single nucleotide polymorphisms (SNP),
more than 100 genes have been implicated in IBD [11].
Studies in twins have not shown a very strong
concordance. The concordance rate for CD in monozygotic twins is between
35-63%, while for UC, it is 16-18%. Concordance rate in dizygotic twins
is around 4%. This suggests a greater role of the environment in IBD
causation. The cold chain hypothesis and the hygiene hypothesis were
formulated to explain the increased incidence of IBD as a by-product of
alteration of the gut microbiota due to refrigeration and increased
cleanliness [12]. Refrigeration altered the bacteria in the diet and
supported the growth of disease causing organisms; while increased
cleanliness, smaller families and less exposure to animals made children
in developed countries more susceptible to IBD. This altered/impaired
immunological tolerance in response to low bacterial load forms the
basis of hygiene hypothesis, wherein alteration between the balance of
Th1 and Th2 helper cells was proposed as a mechanism of increasing IBD
[13].
To summarize, IBD manifests in a genetically
susceptible individual when he/she is exposed to certain environmental
triggers (infections, diet, domestic hygiene, smoking, etc.) which evoke
an aberrant adaptive immune response.
Clinical Presentation
A diagnosis of IBD should always be entertained in
children with persistent (>1 month) or recurrent (>2 in 6 months)
gastrointestinal symptoms. Abdominal pains, chronic diarrhea, rectal
bleeding and weight loss are some of the common symptoms seen in IBD
patients. In children with UC, rectal bleeding, chronic diarrhea and
abdominal pain are more common; while weight loss is a prominent feature
of CD (58% vs 35%). The classic triad of pediatric CD; abdominal
pain, chronic diarrhea and weight loss is seen in only one-fourth of the
cases; 25% of the children may present with only nonspecific symptoms –
vague abdominal discomfort, lethargy and anorexia [2]. Perianal lesions
in the form of skin tags, sentinel piles and fistulae are more common in
CD. Impaired growth velocity and growth failure are more commonly seen
in CD patients. Impairment of growth parameters can precede the
intestinal mucosal lesion by months to years. Extra-intestinal
manifestations of IBD may be the presenting feature in 6-17% of the
patients. Arthropathy, skin manifestations and aphthous stomatitis are
commonly seen. Primary sclerosing cholangitis (PSC) is more commonly
associated with UC [14].
Diagnosis
The diagnosis of IBD is not straightforward. It rests
on an accurate history and thorough clinical examination, supplemented
by a supportive biochemistry, serology, accurate and complete endoscopy
and characteristic histopathology. Radiological examination in the form
of a barium meal, CT/MRI enteroclysis or PET scan may further aid in the
diagnosis.
History and Examination
A complete history should be obtained with regard to
the frequency and type of stools, the presence of blood/pus per-rectum,
and associated abdominal pain, nausea, vomiting, lethargy and weight
loss. Always ask for presence of nocturnal emergency and tenesmus. In
infants with suspected UC, ask about the type of feeds being given to
the child, as allergic colitis is a close differential. Record family
history of IBD and history of antibiotic usage. Look for
extra-intestinal manifestations like joint swelling, oral ulcers, skin
lesions or visual problems. Chart height and weight centiles, including
BMI. Carry out tanner staging for sexual maturity in all pre-pubertal
and pubertal children. Perform abdominal examination for any tenderness,
masses, lumps, or distension. Examine the perianal area for any skin
tags, abscess, sentinel piles or fistulae [15,16].
Investigations
A complete blood count with ESR, liver function tests
(including albumin), iron status and CRP should be done in all cases of
suspected IBD. Stool culture is necessary to rule out infectious
diarrhea. Clostridium difficile toxin should be investigated in a
fresh stool sample, especially if the child has received multiple
antibiotics. However, it is pertinent to note that a documented enteric
infection does not rule out the possibility of IBD [15].
Anemia, thrombocytosis, hypoalbuminemia with
increased ESR and CRP values are expected in patients with IBD. However,
the values may be falsely normal in mild UC (54%) or mild CD (21%).
Serological markers and stool tests
Antibodies to anti-Saccharomyces cerevisiae (ASCA)
are associated with 60% cases of CD; while perinuclear antineutrophil
cytoplasmic antibodies (p-ANCA) are associated with 60% of cases with
UC. As, there is considerable overlap among the antibodies with each
other and for other diseases like tuberculosis, they cannot be used in
isolation to diagnose IBD. Additional markers like anti-E.coli outer
membrane porin C antibody (anti-OmpC), antibodies to bacterial flagellin
(anti-CBir1) and anti-glycan antibodies are being studied [17,18].
Non-invasive stool markers like fecal calprotectin
and lactoferrin are increasingly been recognized as useful markers of
small and large bowel inflammation in IBD patients [19, 20]. Serial
values may be of more benefit than single values as mucosal inflammation
needs time to subside. Values of more than 100-150 µg/g of stool may
differentiate IBD from functional causes. Stool markers need to be
interpreted with caution in settings where invasive enteric infections
are prevalent.
Endoscopy and histopathology
Ileocolonoscopy and upper gastrointestinal endoscopy
(UGI) are absolutely essential for diagnosis of IBD. The EECO and
ESPGHAN guidelines recommend UGI endoscopy even in suspected UC cases to
rule out CD. UGI involvement in CD cases is estimated to vary between
30-80%. Esophageal involvement was seen in 27% cases while
gastro-duodenal involvement in 56% of the cases from the Pediatric IBD
Collaborative research group registry [21-23]. The characteristic
clinical, macroscopic and microscopic findings for CD and UC are given
in Table I.
TABLE I Clinical Differences Between Ulcerative Colitis and Crohn’s Disease
|
Feature |
Crohn’s disease |
Ulcerative colitis |
|
Fever and weight loss |
More common |
Less common |
|
Disease extent |
Anywhere in the GI tract from mouth to anus; rectum is rarely
involved. |
Limited to colorectal mucosa, usually beginning at
the rectum and spreading upwards to the cecum |
|
Inflammation |
Transmural; can lead to fistula. Patchy areas
of inflammation (Skin lesions) |
Mucosali, no fistula. Continous area of inflammation.
|
|
Perianal involvement |
fistulas, anal fissures and skin tags common |
Not as common
|
|
Stenosis |
common |
rare |
|
Feature |
Crohn’s disease |
Ulcerative Colitis |
|
Typical features on endoscopy |
Discontinuous inflammation with intervening normalcy.
Ulceration, structuring and fistulae, Cobblestoning. |
Continuous inflammation with variable proximal extension from
rectum. Erythema, friability and ulceration. Loss of
vascular pattern, pseudopolyp formation |
|
Typical features on histology |
Submucosal/ Transmural inflammation;Chronic ileitis/ colitis;
Non pericrypt granuloma; Focal biopsy changes; Patchy
distribution; Crypt distortion and abscess |
Mucosal inflammationChronic colitis with crypt distortion and
crypt abscess; Goblet cell depletion; Lymphoplasmacytosis;
Plasma cell metaplasia |
The recently adapted Paris classification for
Pediatric IBD, which was derived from the adult Montreal classification,
has elucidated both the macroscopic and microscopic features of UC and
CD in children. As the disease location and disease severity are
determinants of the treatment strategy and the ultimate outcome, a
uniform classification ameliorates any ambiguity in disease
differentiation, phenotype and severity. The Paris classification for CD
and UC are given in Tables II and III, respectively
[21,24].
TABLE II Paris Classification of Crohn’s Disease
|
Age at diagnosis |
A1a |
< 10 years |
|
A1b |
10-<17 years |
|
A2 |
17-40 years |
|
A3 |
> 40 years |
|
Location |
L1 |
Distal 1/3 ileum +/- limited cecal disease |
|
L2 |
Colonic disease |
|
L3 |
Ileocolonic disease |
|
L4 |
Isolated Upper GI disease |
|
L4a |
Esophageal disease |
|
L4b |
Gastroduodenal disease |
|
Behaviour |
B1 |
Non stricturing, nonpenetrating |
|
B2 |
Stricturing |
|
B3 |
Penetrating |
|
B2B3 |
Stricturing and penetrating |
|
P |
Perianal disease modifier |
|
Growth |
G0 |
No evidence of growth delay |
|
Source: Crohn’s & Colitis Foundation of America. |
TABLE III Paris Classification of Ulcerative Colitis
|
Extent |
E1 |
Ulcerative proctitis |
|
E2 |
Left sided colitis distal to splenic flexure |
|
E3 |
Extensive colitis distal to hepatic flexure |
|
E4 |
Pancolitis, proximal to hepatic flexure |
|
Severity |
S0 |
Never severe |
|
S1 |
Ever severe |
|
Source: Crohn’s & Colitis Foundation of America. |
Imaging studies
Fluoroscopy, CT, MRI and nuclear medicine scans are
available to image the bowel in pediatric IBD. The Porto criteria
formulated in 2005 advocated small bowel imaging (Barium meal follow
through) in IBD patients, especially those with CD, to rule out
structuring and fistulae [21]. CT enterography and MR enterography are
emerging as modalities with better resolution and delineation of the
lumen and folds, with MR having less radiation exposure [25]. The use of
PET scan to find areas of increased functional uptake and identify
metabolically active tissue is still experimental. Video capsule
endoscopy (VCE) is helpful in children, where ileal intubation is
unsuccessful or not possible. It is also useful in classifying patients
of IC. The drawbacks include inability to make a tissue diagnosis and
the possibility of a retained capsule in stricturing CD [26-29].
While small bowel imaging using fluroscopy may show
superficial mucosal disease better than any other modality,
extra-luminal disease is poorly visualized. CT scan has greater
resolution and can show extramural disease and its attendant
complications; its use in pediatrics is limited due to the risk
associated with ionizing radiation. MRI is costly and time consuming
when compared to the other modalities, but can be used when soft tissue
characterization is required (perianal CD). Pediatric CT protocols are
now available to limit the total radiation dose being given to children
[30].
In countries and settings where tuberculosis (TB) is
endemic, all efforts should be made by the treating clinician to
distinguish it from CD, which is its closest differential. The fact that
treatment approaches of the two diseases are diametrically opposite
(antibacterials in TB vs immunomodulators in CD), it is all the
more important to differentiate between the two. Colonoscopic features
which suggest CD include perianal lesions, longitudinal ulcers, aphthous
ulcers and cobblestoning. Features suggestive of TB include transverse
ulcers, involvement of fewer colonic segments, a patulous ileocecal
valve and pseudopolyp formation. Radiological features of CD include
symmetric concentric bowel wall thickening with transmural enhancement.
Segmental intestinal stenoses and fistulae formation is nearly always
associated with CD. Extramural features like mesenteric vascular
stranding and fibrofatty proliferation are pathognomonic of CD.
Intestinal TB is characterized by asymmetric bowel wall thickening with
predominant involvement of the ileocecal area and large necrotic
lymphnodes in the mesentry. Tissue diagnosis is mandatory for confirming
either disease. Caseating granulomas are specific for TB while
non-caseating epitheloid cell granulomas are more often found (though
not specific) in CD [31,32]. Fig.1 shows schematic diagram
to evaluate a child with IBD isregretted.
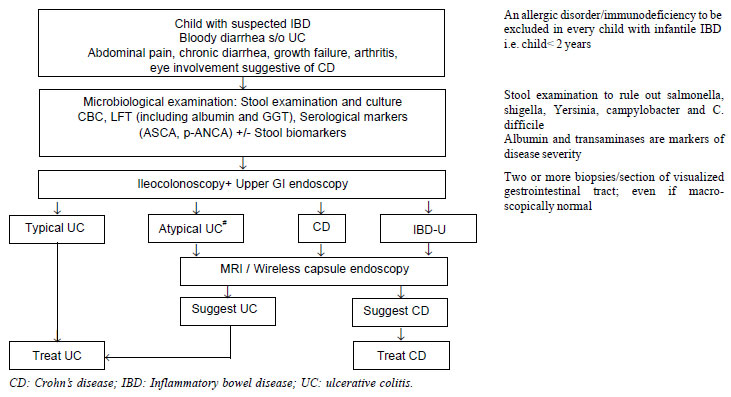 |
|
Fig. 1 Diagnostic algorithm in a child
with suspected IBD (adapted from ESPGHAN Revised Porto Criteria
for the Diagnosis of Inflammatory Bowel Disease in Children and
Adolescents 2014). #Atypical UC includes the following
phenotypes: Rectal sparing, Cecal Patch, UGI involvement, Short
duration, acute severe colitis.
|
Treatment and Monitoring Strategies
The treatment protocols in IBD are aimed at mucosal
healing, with consequent reduction in complications and increased
quality of life. The goals of therapy are to maximize efficacy, minimize
toxicity, prevention of complications, and maintaining/re-establishing
growth velocity and pubertal growth.
The treatment paradigm in pediatric IBD as in the
adult world is the ‘Step- up’ approach, wherein medications with milder
toxicity are used as first line therapy, before moving onto more
aggressive therapies with higher toxicity. The Pediatric Ulcerative
Colitis Activity Index (PUCAI) is a validated score to assess disease
activity in UC. It has the advantage of being non-invasive and can be
calculated easily in clinical practice (Web Table I).
Studies have documented its high correlation with colonoscopy findings
[33,34].
PUCAI score <10 indicates remission; 10-34: mild
disease activity; 35-64: moderate disease activity; >65: severe disease
activity. A clinically significant response to therapy is a fall of more
than 20 points. A similar score known as the Pediatric Crohn’s Disease
Activity index (PCDAI) is available for disease monitoring in CD (Web
Table II). The PCDAI score can range from 0-100, with higher
scores signifying more active disease. A score of <10 is consistent with
inactive disease, 11-30 indicates mild disease, and >30 is moderate-
severe disease. A decrease of 12.5 points is taken as evidence of
improvement.
Ulcerative Colitis
The treatment can be divided into induction of
remission and maintenance. The therapies available to induce remission
include 5-aminosalicylic acid (5-ASA), corticosteroids, anti-tumor
necrosis factor (TNF) therapy and calcineurin inhibitors. The drugs that
can be used to maintain remission include 5-ASA, thiopurines, anti-TNF
therapy and a few selected probiotics.
Most guidelines recommend oral 5-ASA regimes as first
line therapy during induction in mild-to-moderate UC. These are also to
be used as maintenance therapy regardless of other treatments.
Combination of oral and rectal 5-ASA compounds has been shown to be more
effective than an oral drug alone. Topical 5-ASA (enemas) can be used as
monotherapy in children with proctitis alone. Mesalazine and
Sulfasalazine are the 5-ASA agents of choice. A wide variety of ASA
preparations are available in the market including azo-compounds
(sulfasalazine, olsalazine), controlled release (Pentasa), pH-dependent
(Salofalk, Asacol); however, there is no difference in the mucosal
healing rate of the different compounds [35].
Oral steroids (in a single daily dose) are effective
agents in inducing remission in UC; however, they are not to be used in
maintenance phase. These are recommended in moderate UC with systemic
symptoms or severe UC without symptoms, and they can also be used in
children who fail to achieve remission with optimal dose of 5-ASA
agents. The dose of prednisolone is 1-2 mg/kg/day (max: 40mg/day) for
2-4 weeks till remission is achieved. It can then be tapered gradually
over the next 4-8 weeks. Children with severe colitis require
hospitalization with vitals monitoring, complete blood counts and
abdominal X-ray. Intravenous steroids, hydrocortisone (2 mg/kg
four times a day) or methyl prednisolone (2 mg/kg/day), should be given
in such cases. Failure to respond requires rescue therapy with either
intravenous cyclosporine or infliximab [34].
Antibiotics have no role in either induction of
remission or maintenance in UC. Intravenous antibiotics like third
generation cephalosporins and metronidazole can be considered if
infection is suspected, especially in cases of toxic megacolon.
Probiotics (VSL#3 and E.coli Nissle) can be considered as
adjuvant therapy in patients with mild UC and residual activity not
responding to standard therapy.
Immunomodulators (Azathioprine (AZA) and
Mercatopurine (MP)) are indicated only for maintenance of remission. The
scenarios for their potential use include: 5-ASA intolerance, frequently
relapsing disease or steroid dependant disease. They can also be given
after inducing remission with steroids in acute severe colitis. If
calcineurin inhibitors like cyclosporin/ tacrolimus were used in acute
severe colitis, the patients would ultimately need AZA/MP. The
therapeutic effect of the thiopurines is delayed and may take 2-3 months
to reach full effect. Western literature recommends assay of thiopurine
methyltransferase (TPMT) genotype or phenotype to identify child at risk
of myelosuppression [36,37]. However, the facility to measure TPMT is
not available at many centers in low-and middle-income countries. Thus,
regular monitoring of complete blood counts and liver function tests
needs to be done as proxy markers for TPMT activity (2 weekly for the
first 4 weeks, monthly thereafter) till metabolite levels become
available. Pancreatitis is the commonest hypersensitivity reaction which
can occur in 3-4% of all cases. Thiopurines, in conjunction with
biologicals, have also been shown to increase the risk of Non-Hodgkin
lymphoma and Hepatosplenic T-cell lymphoma [35].
Infliximab (IFX) in a dose of 5 mg/kg at 0, 2 and 6
weeks followed by 5 mg/kg 8 weekly for maintenance is the agent of
choice in patients with persistently active or steroid-dependant UC, not
controlled by 5-ASA or steroids. It can also be considered in
steroid-refractory disease. The usage of Adalimumab (ADA) in pediatric
UC is anecdotal and limited to case reports; however, it can be
considered in Infliximab failure or intolerance, prior to colectomy
[38].
Surgery should only be considered in cases of
treatment failure with all first line and second line agents. Surgery
can also be considered in symptomatic children who are on multiple
immunosuppressants and are steroid-dependant. The dose of
immunosuppressants and biologicals need to be optimized before referring
an ambulatory case for surgery. Sometimes changing IFX to ADA can also
prove useful. It can also be considered in cases of toxic megacolon. A
two step procedure (colectomy and pouch formation with ileostomy as the
first step followed by ileostomy closure) is the most commonly performed
surgery. Sometimes a single step procedure (restorative proctocolectomy/
ileo-anal pouch without ileostomy) can be performed in children who are
not on high dose steroids. As with major surgeries, pre-operative
clinical status (malnutrition, hypoalbuminemia, steroids) influence
post-operative disease outcomes [2,39].
Crohn’s Disease
For management of CD, it is helpful to categorize
children into mild, moderate and severe phenotypes based on disease
location, extent and severity. In addition, issues like decreased bone
mass and impaired growth velocity have to be factored into the treatment
regimen.
Induction of remission
Exclusive enteral nutrition (EEN) has been
recommended by ESPGHAN as the modality of choice in inducing remission
in children with luminal CD. While steroids have been conventionally
used, EEN has the obvious advantage of lacking the toxicity of
parenteral steroids. Few studies have shown higher remission rates with
EEN as compared to steroids. EEN is to be given for 6-8 weeks [40,41].
Towards the end of the exclusive feeding period, reintroduction of
regular diet should be started gradually over a period of several
weeks. Factors influencing the use of EEN include the childs’ and
parents’ choice, palatability, compliance and cost. Both polymeric and
elemental feeds are available in the Western market. Various guidelines
have advocated the use of nasogastric tubes and even gastrostomy to meet
the volume required for providing adequate caloric intake (120% of total
caloric requirement/day). If EEN does not induce a clinical response in
two weeks, alternative therapeutic strategies should be employed. EEN is
of questionable significance in children with severe pancolitis, oral
and perianal CD [22].
Oral corticosteroids (prednisolone 1-2 mg/kg/d) can
be used for inducing remission in children with moderate to severe
luminal CD, especially if EEN is not available or not tolerated.
Steroids are helpful in achieving quick clinical remission though only a
small percentage of cases demonstrate mucosal healing on endoscopy [42].
Budesonide has been used in mild to moderate ileo-cecal CD to induce
remission. The drug is known for its high topical activity and low
systemic absorption, by virtue of its affinity to the intestinal
glucocorticoid receptor [43,44]. The steroids are to be given at full
dose for 2-4 weeks followed by gradual tapering over the next 8 weeks.
There is no role of steroids in the maintenance therapy of pediatric CD
[45].
Metronidazole (10-20 mg/kg/day) and ciprofloxacin (20
mg/kg/day) are the two antibiotics utilized for perianal CD, especially
of the fistulizing type. A meta-analysis showed that antibiotics are
superior to placebo in active CD [46]. Metronidazole is thought to be
more efficacious in children with colitis while Ciprofloxacin is
preferred in those with ileitis. Azithromycin and rifaximin are the
other antibiotics that have shown benefit during induction of remission
in mild luminal CD.
Anti-TNF therapy with agents like infliximab (IFX) is
recommended to induce remission in children with steroid refractory CD
and children with active peri-anal fistulizing CD. It can also be
considered in children with high risk of poor outcome (deep ulcerations
on endoscopy, pan-enteric disease, advanced osteoporosis, marked growth
failure, and poor response to adequate initial therapy). The induction
dose is same as for patients with UC [47].
Maintenance of remission
Thiopurines (AZA/ 6MP) are the recommended agents for
maintaining steroid-free remission in children with CD. Patients who
received 6-MP after induction of remission are more likely to remain in
remission, when compared with placebo [48]. These immunomodulators
should be prescribed in full doses from the beginning as they require
8-14 weeks to achieve full efficacy.
Methotrexate can also be used as monotherapy for
maintenance of remission in CD [49]. It can also be used as a second
line drug in children with thiopurine failure [50]. MTX is prescribed in
a dose of 15 mg/m
1. Carvalho RS, Abadom V, Dilworth HP, Thompson R,
Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup
of pediatric IBD. Inflamm Bowel Dis. 2006;12:258-62.
2. Abraham BP, Mehta S, El-Serag HB. Natural history
of pediatric-onset inflammatory bowel disease: a systematic review. J
Clin Gastroenterol. 2012;46:581-9.
3. Martin-de-Carpi J, Rodriguez A, Ramos E, Jimenez
S, Martinez-Gomez MJ, Medina E, et al. Increasing incidence of
pediatric inflammatory bowel disease in Spain (1996-2009): the SPIRIT
Registry. Inflamm Bowel Dis. 2013; 19:73-80.
4. Castro M, Papadatou B, Baldassare M, Balli F,
Barabino A, Barbera C, et al. Inflammatory bowel disease in
children and adolescents in Italy: data from the pediatric national IBD
register (1996-2003). Inflamm Bowel Dis. 2008; 14:1246-52.
5. Heyman MB, Kirschner BS, Gold BD, Ferry G,
Baldassano R, Cohen SA, et al. Children with early-onset
inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium
registry. J Pediatr. 2005;146:35-40.
6. Sauer CG, Kugathasan S. Pediatric inflammatory
bowel disease: highlighting pediatric differences in IBD. Med Clin North
Am. 2010;94:35-52.
7. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den
Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pedia-tric
inflammatory bowel disease: a systematic review of international trends.
Inflamm Bowel Dis. 2011;17:423-39.
8. Sathiyasekaran M, Raju BB, Shivbalan S, Rajarajan
K. Pediatric Crohn’s disease in South India. Indian Pediatr.
2005;42:459-63.
9. Avinash B, Dutta AK, Chacko A. Pediatric
inflammatory bowel disease in South India. Indian Pediatr.
2009;46:639-40.
10. Sathiyasekaran M, Bavanandam S, Sankaranarayanan
S, Mohan N, Geetha M, Wadhwa N, et al. A questionnaire survey of
pediatric inflammatory bowel disease in India. Indian J Gastroenterol.
2014;33:543-9.
11. Biank V, Broeckel U, Kugathasan S. Pediatric
inflammatory bowel disease: clinical and molecular genetics. Inflamm
Bowel Dis. 2007;13:1430-8.
12. Aujnarain A, Mack DR, Benchimol EI. The role of
the environment in the development of pediatric inflammatory bowel
disease. Curr Gastroenterol Rep. 2013;15:326.
13. Timm S, Svanes C, Janson C, Sigsgaard T,
Johannessen A, Gislason T, et al. Place of upbringing in early
childhood as related to inflammatory bowel diseases in adulthood: a
population-based cohort study in Northern Europe. Eur J Epidemiol.
2014;29:429-37.
14. Aloi M, Cucchiara S. Extradigestive
manifestations of IBD in pediatrics. Eur Rev Med Pharmacol Sci.
2009;13:23-32.
15. Bousvaros A, Sylvester F, Kugathasan S, Szigethy
E, Fiocchi C, Colletti R, et al. Challenges in pediatric
inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885-913.
16. Kwon YH, Kim YJ. Pre-diagnostic clinical
presentations and medical history prior to the diagnosis of inflammatory
bowel disease in children. Pediatr Gastroenterol Hepatol Nutr.
2013;16:178-84.
17. Davis MK, Andres JM, Jolley CD, Novak DA, Haafiz
AB, Gonzalez-Peralta RP. Antibodies to Escherichia coli outer membrane
porin C in the absence of anti-Saccharomyces cerevisiae antibodies and
anti-neutrophil cytoplasmic antibodies are an unreliable marker of Crohn
disease and ulcerative colitis. J Pediatr Gastroenterol Nutr. 2007;
45:409-13.
18. Davis MK, Valentine JF, Weinstein DA, Polyak S.
Antibodies to CBir1 are associated with glycogen storage disease type
Ib. J Pediatr Gastroenterol Nutr. 2010;51:14-8.
19. Aomatsu T, Yoden A, Matsumoto K, Kimura E, Inoue
K, Andoh A, et al. Fecal calprotectin is a useful marker for
disease activity in pediatric patients with inflammatory bowel disease.
Dig Dis Sci. 2011;56:2372-7.
20. Chang MH, Chou JW, Chen SM, Tsai MC, Sun YS, Lin
CC, et al. Faecal calprotectin as a novel biomarker for
differentiating between inflammatory bowel disease and irritable bowel
syndrome. Mol Med Rep. 2014;10:522-6.
21. Levine A, Koletzko S, Turner D, Escher JC,
Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria
for the dia-gnosis of inflammatory bowel disease in children and
adol-escents. J Pediatr Gastroenterol Nutr. 2014;58:795-806.
22. Ruemmele FM, Veres G, Kolho KL, Griffiths A,
Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN
on the medical management of pediatric Crohn’s disease. J Crohns
Colitis. 2014;8:1179-207.
23. Turner D, Griffiths AM. Esophageal, gastric, and
duodenal manifestations of IBD and the role of upper endoscopy in IBD
diagnosis. Curr Gastroenterol Rep. 2009;11:234-7.
24. Eszter Muller K, Laszlo Lakatos P, Papp M, Veres
G. Incidence and paris classification of pediatric inflammatory bowel
disease. Gastroenterol Res Pract. 2014; 2014: 904307.
25. Alliet P, Desimpelaere J, Hauser B, Janssens E,
Khamis J, Lewin M, et al. MR enterography in children with Crohn
disease: results from the Belgian pediatric Crohn registry (Belcro).
Acta Gastroenterol Belg. 2013;76:45-8.
26. Gralnek IM, Cohen SA, Ephrath H, Napier A, Gobin
T, Sherrod O, et al. Small bowel capsule endoscopy impacts
diagnosis and management of pediatric inflammatory bowel disease: a
prospective study. Dig Dis Sci. 2012;57:465-71.
27. Hudesman D, Mazurek J, Swaminath A. Capsule
endoscopy in Crohn’s disease: are we seeing any better? World J
Gastroenterol. 2014;20:13044-51.
28. Min SB, Le-Carlson M, Singh N, Nylund CM, Gebbia
J, Haas K, et al. Video capsule endoscopy impacts decision making
in pediatric IBD: a single tertiary care center experience. Inflamm
Bowel Dis. 2013;19:2139-45.
29. North American Society for Pediatric
Gastroenterology Hepatology and Nutrition, Crohn’s and Colitis
Foundation of America, Bousvaros A, Antonioli DA, Colletti RB, et al.
Differentiating ulcerative colitis from Crohn disease in children and
young adults: report of a working group of the North American Society
for Pediatric Gastroenterology, Hepatology, and Nutrition and the
Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr.
2007;44:653-74.
30. Frush DP, Goske MJ. Image Gently: toward
optimizing the practice of pediatric CT through resources and dialogue.
Pediatr Radiol. 2015;45:471-5.
31. Weng MT, Wei SC, Lin CC, Tsang YM, Shun CT, Wang
JY, et al. Seminar Report From the 2014 Taiwan Society of
Inflammatory Bowel Disease (TSIBD) Spring Forum (May 24th, 2014):
Crohn’s disease versus intestinal tuberculosis infection. Intest Res.
2015;13:6-10.
32. Makharia GK, Srivastava S, Das P, Goswami P,
Singh U, Tripathi M, et al. Clinical, endoscopic, and
histological differentiations between Crohn’s disease and intestinal
tuberculosis. Am J Gastroenterol. 2010;105:642-51.
33. Turner D, Hyams J, Markowitz J, Lerer T, Mack DR,
Evans J, et al. Appraisal of the pediatric ulcerative colitis
activity index (PUCAI). Inflamm Bowel Dis. 2009;15:1218-23.
34. Turner D, Travis SP, Griffiths AM, Ruemmele FM,
Levine A, Benchimol EI, et al. Consensus for managing acute
severe ulcerative colitis in children: a systematic review and joint
statement from ECCO, ESPGHAN, and the Porto IBD Working Group of
ESPGHAN. Am J Gastroenterol. 2011;106:574-88.
35. Aloi M, Nuti F, Stronati L, Cucchiara S. Advances
in the medical management of paediatric IBD. Nat Rev Gastroenterol
Hepatol. 2014;11:99-108.
36. Weinshilboum RM, Sladek SL. Mercaptopurine
pharmacogenetics: monogenic inheritance of erythrocyte thiopurine
methyltransferase activity. Am J Hum Genet. 1980;32:651-62.
37. Dubinsky MC, Lamothe S, Yang HY, Targan SR,
Sinnett D, Theoret Y, et al. Pharmacogenomics and metabolite
measurement for 6-mercaptopurine therapy in inflammatory bowel disease.
Gastroenterology. 2000; 118:705-13.
38. Yang LS, Alex G, Catto-Smith AG. The use of
biologic agents in pediatric inflammatory bowel disease. Curr Opin
Pediatr. 2012;24:609-14.
39. Baillie CT, Smith JA. Surgical strategies in
paediatric inflammatory bowel disease. World J Gastroenterol.
2015;21:6101-16.
40. Berni Canani R, Terrin G, Borrelli O, Romano MT,
Manguso F, Coruzzo A, et al. Short- and long-term therapeutic
efficacy of nutritional therapy and corticosteroids in paediatric
Crohn’s disease. Dig Liver Dis. 2006;38:381-7.
41. Whitten KE, Rogers P, Ooi CY, Day AS.
International survey of enteral nutrition protocols used in children
with Crohn’s disease. J Dig Dis. 2012;13:107-12.
42. Bousvaros A. Mucosal healing in children with
Crohn’s disease: appropriate therapeutic goal or medical overkill?
Inflamm Bowel Dis. 2004;10:481-3.
43. Escher JC, European Collaborative Research Group
on Budesonide in Paediatric IBD. Budesonide versus prednisolone for the
treatment of active Crohn’s disease in children: a randomized,
double-blind, controlled, multi-centre trial. Eur J Gastroenterol
Hepatol. 2004;16:47-54.
44. Levine A, Weizman Z, Broide E, Shamir R, Shaoul
R, Pacht A, et al. A comparison of budesonide and prednisone for
the treatment of active pediatric Crohn disease. J Pediatr Gastroenterol
Nutr. 2003;36:248-52.
45. Dimakou K, Pachoula I, Panayotou I, Stefanaki K,
Orfanou I, Lagona E, et al. Pediatric inflammatory bowel disease
in Greece: 30-years experience of a single center. Ann Gastroenterol.
2015;28:81-6.
46. Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A,
Marshall JK, et al. Antibiotic therapy in inflammatory bowel
disease: a systematic review and meta-analysis. Am J Gastroenterol.
2011;106:661-73.
47. Olbjorn C, Nakstad B, Smastuen MC, Thiis-Evensen
E, Vatn MH, Perminow G. Early anti-TNF treatment in pediatric Crohn’s
disease. Predictors of clinical outcome in a population-based cohort of
newly diagnosed patients. Scand J Gastroenterol. 2014;49:1425-31.
48. Markowitz J, Grancher K, Kohn N, Lesser M, Daum
F. A multicenter trial of 6-mercaptopurine and prednisone in children
with newly diagnosed Crohn’s disease. Gastroenterology.
2000;119:895-902.
49. Uhlen S, Belbouab R, Narebski K, Goulet O,
Schmitz J, Cezard JP, et al. Efficacy of methotrexate in
pediatric Crohn’s disease: a French multicenter study. Inflamm Bowel
Dis. 2006;12:1053-7.
50. Turner D, Grossman AB, Rosh J, Kugathasan S,
Gilman AR, Baldassano R, et al. Methotrexate following
unsuccessful thiopurine therapy in pediatric Crohn’s disease. Am J
Gastroenterol. 2007;102:2804-12.
51. Sunseri W, Hyams JS, Lerer T, Mack DR, Griffiths
AM, Otley AR, et al. Retrospective cohort study of methotrexate
use in the treatment of pediatric Crohn’s disease. Inflamm Bowel Dis.
2014;20:1341-5.
52. Hyams J, Crandall W, Kugathasan S, Griffiths A,
Olson A, Johanns J, et al. Induction and maintenance infliximab
therapy for the treatment of moderate-to-severe Crohn’s disease in
children. Gastroenterology. 2007;132:863-73;.
53. Nobile S, Gionchetti P, Rizzello F, Calabrese C,
Campieri M. Mucosal healing in pediatric Crohn’s disease after anti-TNF
therapy: a long-term experience at a single center. Eur J Gastroenterol
Hepatol. 2014;26:458-65.
54. Assa A, Hartman C, Weiss B, Broide E, Rosenbach
Y, Zevit N, et al. Long-term outcome of tumor necrosis factor
alpha antagonist’s treatment in pediatric Crohn’s disease. J Crohns
Colitis. 2013;7:369-76.
55. Ford AC, Kane SV, Khan KJ, Achkar JP, Talley NJ,
Marshall JK, et al. Efficacy of 5-aminosalicylates in Crohn’s
disease: systematic review and meta-analysis. Am J Gastroenterol.
2011;106:617-29.
56. Griffiths A, Koletzko S, Sylvester F, Marcon M,
Sherman P. Slow-release 5-aminosalicylic acid therapy in children with
small intestinal Crohn’s disease. J Pediatr Gastroenterol Nutr.
1993;17:186-92.
57. Cezard JP, Munck A, Mouterde O, Morali A,
Lenaerts C, Lachaux A, et al. Prevention of relapse by mesalazine
(Pentasa) in pediatric Crohn’s disease: a multicenter, double-blind,
randomized, placebo-controlled trial. Gastroenterol Clin Biol.
2009;33:31-40.
58. Lin MV, Blonski W, Lichtenstein GR. What is the
optimal therapy for Crohn’s disease: step-up or top-down? Expert Rev
Gastroenterol Hepatol. 2010;4:167-80.
59. Kim MJ, Lee JS, Lee JH, Kim JY, Choe YH.
Infliximab therapy in children with Crohn’s disease: a one-year
evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies.
Acta Paediatr. 2011;100:451-5.
60. Krupoves A, Mack DR, Seidman EG, Deslandres C,
Bucionis V, Amre DK. Immediate and long-term outcomes of corticosteroid
therapy in pediatric Crohn’s disease patients. Inflamm Bowel Dis.
2011;17:954-62.
61. Mayberry JF, Lobo A, Ford AC, Thomas A. NICE
clinical guideline (CG152): the management of Crohn’s disease in adults,
children and young people. Aliment Pharmacol Ther. 2013;37:195-203.

