|
|
|
Indian Pediatr 2016;53: 967 -8976 |
 |
Vitamin D Supplementation for Treatment and
Prevention of Pneumonia in Under-five Children:
A Randomized
Double-blind Placebo Controlled Trial
|
|
Piyush Gupta, Pooja Dewan, Dheeraj Shah, Nisha
Sharma, Nidhi Bedi, *Iqbal R Kaur, $Ajay
Kumar Bansal and #SV
Madhu
From the Department of Pediatrics; *Department of
Microbiology; $Department of Biostatistics and Medical Informatics; and
#Division of Endocrinology, Department of Medicine; University College
of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
Correspondence to: Dr Piyush Gupta, Professor of
Pediatrics, University College of Medical Sciences and Guru Teg Bahadur
Hospital, Dilshad Garden, Delhi 110 095, India.
Email:
[email protected]
Received: June 21, 2016;
Initial review: July 25, 2016;
Accepted: August 11, 2016.
Trial Registration: CTRI/2013/01/003317
|
Objective: To evaluate the
efficacy of single oral mega-dose of Vitamin D3 for treatment and
prevention of pneumonia in under-five children.
Design: Randomized, double blind,
placebo-controlled trial.
Setting: Tertiary-care hospital.
Participants: 324 children (of
980 assessed) between 6 mo-5 y age (median (IQR): 12 (7,19.8) mo) with
WHO-defined severe pneumonia. Of these, 126 (39%) were vitamin D
deficient (serum 25(OH)D <12 ng/mL).
Intervention: 100,000 IU of oral
cholecalciferol (n= 162) or placebo (n= 162) in single
dose, administered at enrolment.
Outcome variables: Primary:
Time to resolution of severe pneumonia and proportion of children having
recurrence of pneumonia in next 6 months; Secondary: Change in
serum levels of 25(OH)D; immunoglobulins IgA, IgG, IgM, and cathelicidin
2 weeks following supplementation; and time taken for overall resolution
of illness.
Results: Median (95% CI) time for
resolution of severe pneumonia was 30 (29, 31) h in the vitamin D group
as compared to 31 (29,33) h in the placebo group [adjusted hazard ratio
(95% CI): 1·39 (1·11, 1·76); P=0·005]. The risk of recurrence of
pneumonia in next 6 months was comparable in the two groups [placebo:
36/158 (22·8%); vitamin D: 39/156 (25%); RR (95% CI): 1·13 (0·67,1·90);
P=0·69]. Proportion of vitamin D deficient children declined from
38% to 4% in the supplementation group, and from 41% to 33% in the
placebo group, two weeks after supplementation. There was no significant
effect of vitamin D supplementation on serum levels of cathelicidin, IgA
and IgG. The time taken for complete recovery from pneumonia, duration
of hospitalization, and fever clearance time were comparable for the two
groups. No adverse event was noted related to the intervention.
Conclusion: There is no robust
evidence of a definite biological benefit, either for therapy or
prevention, to suggest a routine megadose supplement of vitamin D3 for
under-five children with severe pneumonia.
Keywords: Cholecalciferol, LRTI, Micronutrient
Therapy, Prevention, Outcome.
|
|
P
neumonia remains the leading cause of childhood
mortality accounting for 15% of all deaths in children below 5 years of
age [1]. Observational studies have shown an association between vitamin
D deficiency and respiratory tract infections, probably due to its
immune-enhancing properties [2-13]. However, results of only few trials
are available to document the efficacy of vitamin D supplementation on
the incidence, severity, and recurrence of acute respiratory tract
infections in under-five children [14-16]. Maneski-Holland, et al.
[14] observed that administration of 100,000 IU of vitamin D to children
(136 mo) with pneumonia made no difference to recovery but reduced the
risk of repeat episode within 90 days. Another study [15] by the same
group investigated the effect of 100,000 IU vitamin D3
given orally once every 3 months for 18 months to healthy infants aged
111 months, and reported no significant difference between the
incidence of first or only episode of radiologically confirmed pneumonia
between the study and control groups. Another trial, conducted in India
[16] by our group, evaluated oral vitamin D (1000 IU <1 year, 2000 IU >1
year) for 5 days to children 15 years with a clinical diagnosis of
severe pneumonia and observed no difference in time to resolution of
severe pneumonia, between the groups. A head on comparison or
meta-analysis of these trials, though attempted [17], has limited
validity because of substantial variability in the vitamin D dosing,
definition of pneumonia, outcome measures, and duration of follow-up
between various trials. Further, none of these trials documented the
vitamin D level and immune status of the participants, either at
baseline or after supplementation.
We planned to study the role of a single mega dose
(100,000 IU) of oral vitamin D supplementation for treatment and
prevention of community-acquired pneumonia along with estimation of
baseline and post intervention serum 25(OH)D levels, and certain immune
markers. The primary objectives were to document its effect on time to
resolution of severe pneumonia and recurrence of pneumonia in
next six months.
Methods
Study design: This was a
randomized, double blind placebo controlled trial conducted at a
tertiary care hospital in New Delhi, India. Approval was obtained from
the institutional ethical committee of the University College of Medical
Sciences, Delhi. Informed written consent was taken from the caregivers.
Participants: Children aged 6 months to 5
years with a clinical diagnosis of severe pneumonia (defined as presence
of lower chest indrawing in children presenting with cough or difficult
breathing) [18] were included in the study. It was ensured that the
family was staying within a 10 km radius of the hospital. Children
having a history or clinical features suggestive of rickets (presence of
wide wrists, delayed closure of anterior fontanel, presence of rachitic
rosary, bow legs or knock knee), severe acute malnutrition, asthma,
hypertension, complicated pneumonia (lung abscess, pleural effusion,
empyema) or illness severe enough to require ventilation, chronic
respiratory disease, heart disease, renal or hepatic insufficiency,
neurological illness resulting in abnormalities of muscle tone/power,
and known immunodeficiency were excluded. Children having received
vitamin D or calcium supplements within four weeks prior to enrolment,
those diagnosed with hypercalcemia or allergy to vitamin D, or immunized
with pneumococcal/flu vaccine were also excluded.
Randomization and masking: Eligible
children were randomized using computer-generated block randomi-zation
to receive 100,000 IU of vitamin D (cholecalciferol) or placebo orally.
Eight, ten, and twelve blocks consisting of 10, 10, and 12 subjects,
respectively were created. The drug and placebo were manufactured and
supplied by M/s Zuventus Healthcare Ltd., India, in granule form, packed
as 60,000 IU per sachet. The amount of vitamin D in the supplement was
not determined independently from that described on the sachet. Both
drug and placebo were identical in appearance, color, odor, amount, and
taste. Five sachets of the drug were weighed and repackaged into three
airtight zip pouch containing 100,000 IU of cholecalciferol each with
the help of electronic weighing scale (0·001 g calibration). Placebo was
also processed in similar manner. Only 15 doses were prepared at a time.
Both drug and placebo were stored in a cool, dry, and dark place till
dispensed. The next lot was prepared afresh when 4 doses were left. The
allocation was further concealed by using sealed opaque envelopes.
Randomization, repackaging, sequencing, and allocation concealment were
done independently by a bio-statistician and an office secretary who
were not members of the investigating team. None of the investigators,
study staff, and participants was aware of the drug or placebo being
dispensed. The codes were revealed only at the time of final data
analysis.
Baseline data-collection: Details were recorded
for socio-demographic variables (age, sex, socio-economic status,
feeding practices), immunization status, nature and duration of
presenting symptoms, and past history of similar episodes/nebulization.
All children were examined for vital signs (temperature, heart rate,
respiratory rate, blood pressure, oxygen saturation), pallor, cyanosis,
nasal flaring, grunt, and mental status. Respiratory rate was measured
for a full minute and if fast (RR >50/min for 6 months1 year and
>40/min for 15 years) [18,19], it was measured again and the two
readings were averaged. The count was done at a time when the child was
quiet. Axillary temperature was measured using a standard mercury
thermometer. Fever was defined as temperature
³38°C. Baseline
oxygen saturation was measured using a pulse oximeter with a probe on a
finger or toe, in room air. Chest was auscultated for presence of any
added sounds (wheeze and/or crepitations). Weight, length/height,
mid-upper arm circumference, and head circumference were recorded for
all participants as per standard techniques [20]. Weight-for-age Z-score
(WAZ), height/length-for-age Z-score (HAZ), and
weight-for-height/length Z-score (WHZ) were derived using WHO
Anthro software [21]. This software uses WHO reference standards for
growth of under-5 children [22].
Intervention: A single dose of 100,000 IU of
vitamin D (cholecalciferol) was dissolved in milk and administered
orally or by nasogastric tube to the participant, on the day of
enrolment after collection of the blood samples. Participants were
treated as per a standard protocol. On admission, measures were taken to
establish and maintain a patent airway, breathing, and circulation.
Hydration was maintained and intravenous fluids were administered if
oral intake was poor. Oxygen was provided with a face mask or oxygen
hood, if the child was having marked respiratory distress, signs of
hypoxia, or oxygen desaturation. The child was nebulized with salbutamol
if there was evidence of bronchospasm (presence of wheeze) or fast
breathing with past history of nebulization. Blood samples were drawn
only after the initial stabilization and before administration of
drug/placebo. Antibiotics were administered for severe pneumonia as per
the guidelines of the Indian Academy of Pediatrics [23].
Hospital follow-up: Children were monitored and
recorded every eight hourly for respiratory rate, chest indrawing,
oxygen saturation, auscultation findings, fever, feeding, cyanosis, and
mental status. Child was re-classified from severe pneumonia to
pneumonia when lower chest indrawing disappeared (whereas fast breathing
persisted), and remained absent for next 24 hours. The child was
discharged when fever and fast breathing were absent for at least 24
hours.
Home follow-up: At home, participants were
followed for 180 days (from day of enrolment) to document the recurrence
of episodes of pneumonia. Field workers made home visits every fortnight
starting from the day of discharge and enquired about episodes of cough
or/and difficult breathing. An episode of cough associated with
fast/difficult breathing (as reported by the mother) which warranted
medical attention was regarded as an episode of pneumonia. The severity
of pneumonia was not graded as the child was assessed by the field
worker only at home. Wherever available, the records of hospitalization/
treatment were reviewed. An episode was regarded as recurrence if the
child remained free of symptoms of cough or fast breathing for at least
seven days following completion of the course of antibiotic therapy as
per protocol for the previous episode of pneumonia.
Investigations: Baseline hemoglobin, total
leucocyte count, platelet count, blood culture, and chest X-ray
were performed in all subjects at enrolment, as part of routine work-up.
A 3 mL venous blood sample was obtained in a serum separator vacutainer
at enrolment, at 14 days, and 3 months after enrolment. Serum were
separated and stored after labeling them appropriately at 20ΊC in a
freezer. Blood samples for 25-hydroxyvitamin D and parathyroid hormone
(PTH) were collected and transported in ice. Serum vitamin D (25(OH)D),
Parathormone, serum calcium, serum phosphorous, and serum alkaline
phosphatase were estimated in all three samples, in all participants.
Immunological markers (serum immuno-globulin IgA, IgG, IgM, and
cathelicidin anti-microbial peptide (CAMP)) were estimated at enrolment
and after 14 days; initially in all subjects, and later in alternate
participant.
Laboratory procedures: Serum PTH and serum
25(OH)D were estimated by radioimmunoassay (RIA) using commercially
available kit manufactured by Immunotech SAS, France (interassay
variation: below or equal to 10·3%; intra-assay variation: below or
equal to 7·7%; sensitivity: 2 pg/mL) and DiaSorin, USA (interassay
variation: 11%; intra-assay variation: 12·5%; sensitivity: at or below
1·5 ng/mL), respectively. Cathelicidin anti-microbial peptide (CAMP) in
serum was estimated using a standard commercial kit (Human LL-37, HK 321
Hycult Biotech, Netherlands (sensitivity: 0·1 ng/mL), based on sandwich
enzyme immunoassay (ELISA), as per manufacturers instructions. Serum
immunoglobulins (IgA, IgG and IgM) were measured quantitatively with
immunoenzymatic colorimetric method using ELISA based kits (Xema Co Ltd,
Russia) having a sensitivity of 0·12 g/L.
Outcomes: The primary outcome variables were (a)
the time to resolution of severe pneumonia (the duration from the
enrolment till the chest indrawing was no longer present, and continued
to be absent for next 24 hours); and (b) the proportion of
children having a recurrence of pneumonia in next six months. The
secondary outcome variables included change in the serum level of
25(OH)D and
PTH after two weeks and three months of therapy;
change in serum level of cathelicidin and immuno-globulins (IgA, IgG,
IgM) after two weeks of therapy; duration of hospitalization; time to
complete recovery from pneumonia (normalization of respiratory rate),
fever clearance time; and incidence rate of pneumonia during follow-up.
All primary and secondary outcome measures were also studied in vitamin
D deficient participants (serum 25(OH)D
<12 ng/mL) [24].
Safety and adverse events: All recruited patients
were assessed for clinical evidence of vitamin D intoxication in the
first week after administering drug/placebo. Symptoms pertaining to
hypervitaminosis such as dehydration, vomiting, decreased appetite
(anorexia), irritability, constipation, fatigue, abdominal cramps,
muscle weakness, and polyuria were enquired. Blood pressure was measured
routinely for any evidence of hypertension. Biochemically, participants
were monitored for presence of hypercalcemia (serum calcium greater than
10·8 mg/dL) [25] at two weeks as a sign of toxicity. Serum calcium above
14 mg/dL was set as the cut-off for treatment with intravenous
furosemide and pamidronate (bisphosphonates) [26]. All adverse events
occurring during 6 months follow-up period were also recorded.
Statistical Analysis
Sample size: Sample size was calculated for both
primary outcomes, using data from a study by Manaseki-Holland, et al.
[14] A sample size of 104 children in each group was considered adequate
to detect a difference of 24 hours in time to resolution of severe
pneumonia between the vitamin D (SD 2·22) and placebo (SD 2·89) groups,
with 80% power and a
= 0·05. To account for 10% attrition, the minimum required sample size
was 115 for each group. For the second outcome measure, a sample size of
162 children in each group was required to detect a 30% relative
reduction in proportion of children suffering from a repeat episode of
pneumonia in the next six months in the vitamin D supplemented group,
accounting for 20% attrition.
Statistical methods: Cox proportional hazards
regression model was constructed to create the time to event curves and
estimate the hazard ratio (HR) with 95% confidence interval (95% CI)
between the treated and control groups for time to resolution of severe
pneumonia, and adjusted for co-variates. Hazard ratio >1 indicates the
relative likelihood of disease resolution in treated versus
control subjects at any given point in time. Covariates included age,
sex, nutritional status (WHZ score), severity of illness (respiratory
rate), and baseline serum 25(OH)D levels. Incidence of pneumonia during
follow up was calculated by dividing the total number of new episodes of
pneumonia by total time at risk, for all children in each group.
Relative risk for incidence of recurrence of pneumonia (the second
primary outcome variable) was compared between the groups.
Changes (pre-post) in biochemical and immuno-logical
markers between the groups were compared by unpaired Student t
test. Parameters which did not follow a normal distribution were
log-transformed. Non-parametric (Mann Whitney U) test was used to
compare groups if the applied transformation did not result in normal
distribution. Within group means at baseline and follow-up were compared
with paired t-test; or Wilcoxon signed rank test, if the data were not
normally distributed. Kaplan-Meier survival function plots were
constructed to compare the median duration for time to complete recovery
from pneumonia, fever clearance time, and duration of hospitalization,
between the two groups (placebo and vitamin D supplemented) by using the
log rank test. P<0·05 was considered as significant. Bonferroni
correction was applied to keep the type 1 error as 5% in total. The
above analyses were also conducted for the subgroup of vitamin D
deficient participants.
The effect of vitamin D supplementation on outcome
variables was analyzed on an intention-to-treat basis. The data were
analyzed by using SPSS software version 20·0.
A data and safety monitoring board (DSMB) reviewed
and evaluated the accumulated study data on yearly basis for participant
safety, study conduct, data management, and progress.
Results
The participants were screened for recruitment
between August 25, 2012 to January 27, 2015. Trial profile is depicted
in Fig. 1. Randomized children (n=324) included 10
trial deviates (7 with rickets and 3 with heart disease) who were missed
in the initial screening.
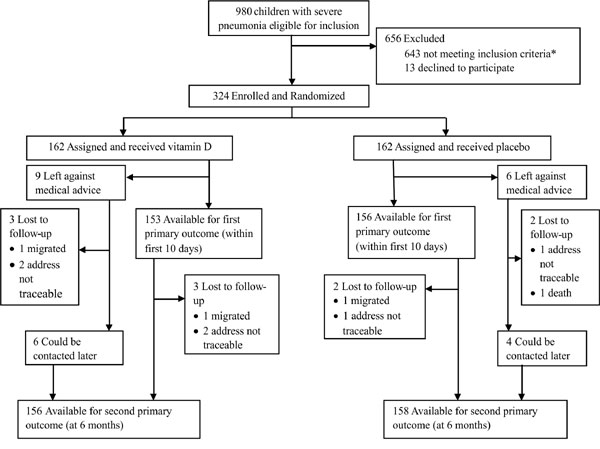 |
|
*Reasons for exclusion (n): residence
more than 10 km away from the hospital (53); received vitamin D
or calcium supplements (22); weight for height Z-score <-3 (35);
rickets (62); history of nebulization on 3 or more occasions
(191); congenital heart disease (84); chronic respiratory
disease (tuberculosis) (17); neurological illness: (46);
renal/hepatic insufficiency (6); anemia requiring transfusion:
(9); complicated pneumonia (55); severe illness requiring
ventilator care (63).
Fig.1 Trial profile showing
participant enrolment.
|
The median (IQR) age of enrolled children (226
(69·8%) boys) was 12 (7,20) months. Number of children in age categories
of 6 m1 y, 12 y, and >2 y were 186 (57·4%), 75 (23·2%), and 63
(19·4%), respectively. Of the 324 participants, 121 (37·3%) children had
WHZ scores between 1 to 2 SD, followed by 89 (27·5%) participants in
median to 1 SD. 69/324 (21·3%) participants had WHZ score between 2 to
3 SD. Similar trends, with maximum number of participants in 2 to 3
SD category, were observed for weight-for-age Z-score (118/324) and
length-for-age Z-score (103/324). Baseline socio-demo-graphic and
clinical characteristics of the participants in the two study arms are
compared in Table I.
TABLE I Baseline Characteristics of Study Participants (N= 324)
|
Variable |
Vitamin D supplemented |
Placebo group |
|
group (N=162) |
(N=162) |
|
Age (mo), mean (SD) |
16·4 (12·9) |
16·9 (13·4) |
|
Male: Female |
113:49 |
113:49 |
|
Socioeconomic class, n (%) |
|
Upper |
3 (1·9) |
2 (1·2) |
|
Middle |
51 (31·5) |
39 (24·0) |
|
Upper lower |
108 (66·7) |
121 (74·7) |
|
Received exclusive breastfeeding for 6 months n, (%) |
98 (60·5) |
99 (61·1) |
|
Total duration of breastfeeding (months) [Mean (SD)] |
11 (7·0) |
10 (6·7) |
|
History of similar episode in past/nebulization [n (%)] |
70 (43·2) |
59 (36·4) |
|
Children unimmunized n (%) |
14 (8·6) |
11 (6·8) |
|
Nutritional Status |
|
Weight (kg) |
8·5 (2·38) |
8·6 (2·60) |
|
Weight for age Z-score (WAZ) |
1·7 (0·95) |
1·6 (1·01) |
|
Height for age Z-score (HAZ) |
1·4 (1·19) |
1·5 (1·14) |
|
Weight for height Z-score (WHZ) |
1·2 (1·11) |
1·1 (1·07) |
|
Body mass index |
15·2 (1·39) |
15·3 (1·54) |
|
Mid upper arm circumference (cm) |
13·3 (1·10) |
13·4 (1·1) |
|
Duration of illness (d), mean (SD) |
|
Fever |
3 (1·6) |
3 (1·6) |
|
Breathlessness |
1 (0·9) |
1·4 (0·8) |
|
Vital signs, mean (SD) |
|
|
|
Respiratory rate (per minute) |
62 (8) |
62 (8) |
|
Oxygen saturation (%) |
96 (3) |
96 (3) |
|
Physical signs, n (%) |
|
Pallor |
41 (25·3) |
43 (26·5) |
|
Nasal flaring |
115 (71·0) |
114 (70·4) |
|
Grunt |
1 (0·6) |
0 (0) |
|
Wheeze |
137 (84·6) |
127 (78·4) |
|
Crepitations |
158 (97·5) |
162(98·8) |
|
Clinical diagnosis on admission, n (%) |
|
Bronchopneumonia |
98 (60·5) |
100 (61·7) |
|
Bronchiolitis |
22 (13·6) |
21 (13·0) |
|
Wheeze associated respiratory tract infection |
42 (25·7) |
41 (25·3) |
The prevalence of anemia (Hb <11 g/dL) in the study
population was 267/324 (82·4%); hypocalcemia (calcium <8·8 mg/dL) and
hypophosphatemia (serum phosphorus <3·8 mg/dL) were observed in 55·9%
(180/322) and 31·4% (101/322) participants, respectively. Raised serum
alkaline phosphatase (>283 IU/L for 1-12 months, >345 IU/L for 13-36
months, and >309 IU/L for > 37 months age) was documented in 47
participants. The baseline hematological, biochemical, hormonal and
immunological parameters between the two groups are compared in
Web Table I. Vitamin D deficiency (serum 25(OH)D
<12 ng/mL) was present in 61/162 (37·6%) children in vitamin D
supplemented group compared to 65/162 (40·1%) in the placebo group.
Blood culture was positive in 28 (8·6%) children, of which
Staphylococcus aureus was isolated in 27 cases. Baseline chest X-ray
was abnormal in 292 (90·1%) children. Consolidation or bilateral patchy
opacities were observed in 14 children, while the rest had
hyperinflation and/or minor infiltrates.
Web Fig. 1a shows resolution of severe
pneumonia as survival curves for the two groups, after adjusting for
covariates. Median time taken for resolution of severe pneumonia was 30
(95% CI 29, 31) h in the vitamin D group as compared to 31 (95% CI 29,
33) h in the placebo group. The unadjusted hazard ratio for resolution
of severe pneumonia in treated vs control subjects at any given
point of time was 1·31 (95% CI 1·04, 1·64; P=0·020). The
difference was further adjusted for age, sex, respiratory rate at
enrolment (for severity of illness), weight-for-height Z-score
(nutritional status), and serum 25(OH)D levels. The relative likelihood
of resolution of severe pneumonia in vitamin D supplemented group
remained significantly higher after adjusting for respiratory rate and
the rest of covariates (adjusted hazard ratio: 1·39 (95% CI 1·11,1·76;
P=0·005). This translated to a 58% (95% CI 5264%) chance of the
patient having earlier resolution of severe pneumonia.
The proportion of children with recurrence of
pneumonia in 6 months following supplementation and the number of
children having multiple (>1) episodes of recurrence of pneumonia was
comparable in the two groups (Table II). The risk of a
repeat episode of pneumonia within 6 months of supplementation was
comparable between the two groups (placebo: 36/158 (22·8%); vitamin D:
39/156 (25%); relative risk: 1·13 (95% CI 0·671·90; P=0·69). The
incidence of recurrence of pneumonia for children having received
vitamin D was 0·056 episodes per month; compared to 0·052 episodes per
month for children in the placebo group.
TABLE II Recurrence of Pneumonia in 6 Months Following the Resolution of the Initial Episode
|
Variable |
Supplementation group |
P value |
|
Vitamin D |
Placebo |
|
|
(n=156) |
(n=158) |
|
|
Recurrence of pneumonia, n (%) |
39(25) |
36 (22.8) |
0·64 |
|
1 episode |
29 |
27 |
|
|
2 episode |
7 |
6 |
|
|
3 episode |
3 |
2 |
|
|
4 episode |
0 |
1 |
|
Table III compares the secondary outcome
measures between the two study groups. Number of vitamin D deficient
children in the vitamin D supplemented group declined from 61/162
(37·6%) to 6/151 (4%), and 15/144 (10·4%), after 2 weeks and 3 months of
follow-up, respectively. In the placebo group, the corresponding
proportion of vitamin D deficiency was 40·1%, 33·3%, and 36·2%,
respectively at baseline, 2 weeks, and 3 months. Duration of
hospitalization, time taken for recovery from pneumonia, and time taken
for resolution of fever were comparable between the two groups (Web
Fig.1b-d).
TABLE III Secondary Outcomes in the Two Study Groups
|
Secondary Outcomes |
Supplementation Group |
P value |
|
N |
Vitamin D |
N |
Placebo |
|
|
Change in serum 25 (OH) vitamin D (ng/mL), mean (SD) |
|
From baseline to 2 weeks |
151 |
30·1 (27·1) |
144 |
1·9 (14·7) |
<0·001 |
|
From baseline to 3 months |
144 |
7·0 (18·8) |
138 |
0·2 (16·9) |
0·002 |
|
Change in serum PTH (pg/mL), mean (SD) |
|
From baseline to 2 weeks |
151 |
14·9 (53·1) |
144 |
0·5 (42·3) |
0·006 |
|
From baseline to 3 months |
144 |
8·2 (56·2) |
138 |
1·9 (59·0) |
0·36 |
|
Change in serum cathelicidin (ng/mL), mean (SD) |
|
From baseline to 2 weeks |
74 |
1·5 (14·7) |
76 |
1·6 (8·4) |
0·12 |
|
Change in serum IgA (mg/dL), mean (SD) |
|
From baseline to 2 weeks |
119 |
0·1 (0·5) |
122 |
0·1 (0.4) |
0·43 |
|
Change in serum IgG (mg/dL), mean (SD) |
|
From baseline to 2 weeks |
119 |
0·6 (2·9) |
122 |
0·2 (1·8) |
0·26 |
|
Change in serum Ig M (mg/dL), mean (SD) |
|
From baseline to 2 weeks |
119 |
0·3 (1·7) |
122 |
0·4 (1·7) |
0·92 |
|
Duration of hospitalization (h), mean (SD) |
152 |
104·7 (37·9) |
156 |
109·4 (46·0) |
0·32 |
|
Time to complete recovery from pneumonia (h), mean (SD) |
153 |
48·8 (25·0) |
156 |
50·8 (29·5) |
0·93* |
|
Fever clearance time (h), mean (SD) |
78 |
20·7 (18·2) |
80 |
18·1 (14·1) |
0·50* |
|
P value computed on means and *log transformed means by
unpaired t-test. |
Only 9/324 (2·8%) children required re-dosing of the
supplementation (6 in placebo group and 3 in vitamin D group). Of these,
five children (four in placebo and one in vitamin D group) had a single
episode of vomiting immediately after ingestion and rest of the children
spilled the content.
No adverse reaction were noted in any of the study
participants following supplementation. Hypercalcemia (serum calcium
greater than 10·8 mg/dL) at 2 weeks was not noted in any of the
participant.
Mean duration of follow-up for 318 participants (home
address of 6 participants were not traceable) was 23·8 weeks. Numbers of
adverse events during follow-up in the two groups are compared in
Table IV. All the adverse events happened after 4 weeks
of administration of intervention. There was no death during
hospitali-zation or within first 14 days of enrolment. One death was
reported after 28 days of enrolment by the field staff. This child,
belonging to the placebo group, left without information on day 2 of
hospitalization. The field staff came to know that the child died 26
days later in the village.
TABLE IV Adverse Events During Follow-up of 6 Months
|
Adverse events |
Supplementation group |
|
Vitamin D |
Placebo |
|
(n=156) |
(n=159) |
|
Adverse events*, n(%) |
54 (34.6) |
49 (30.8) |
|
One |
45 |
42 |
|
Two |
7 |
7 |
|
Three |
2 |
0 |
|
Serious adverse events, n(%) |
19 (12.2) |
20 (12.6) |
|
Serious adverse events |
4 (2.6) |
2 (1.3) |
|
other than pneumonia, n (%) |
|
*Other than pneumonia and acute upper respiratory infections. |
We also compared the outcome measures for vitamin D
deficient participants (serum 25(OH)D <12 ng/mL; n=126/324
(38·9%), in a sub-group analysis. For estimation of time to resolution
of severe pneumonia, 116/126 (92·1%) vitamin D deficient participants
were available (56/61 (91·8%) in vitamin D group and 60/65 (92·3%) in
placebo group). Median time for resolution of severe pneumonia was 30 h
(95% CI 2733 h) in the vitamin D group as compared to 32 h (95% CI
2440 h) in the placebo group (unadjusted hazard ratio 1·46 (95% CI
1·01, 2·12); P=0·047). The difference was adjusted for age, sex,
nutritional status, respiratory rate and baseline serum 25(OH)D levels.
The relative likelihood of resolution of severe pneumonia in vitamin D
group remained significantly higher (adjusted HR: 1·66 (95% CI 1·12,
2·45); P=0·011). The risk of a repeat episode of pneumonia within
6 months of supplementation in vitamin D deficient children was
comparable in the two groups (placebo: 14/63 (22·2%); vitamin D: 11/58
(19%); relative risk: 0·82 (95% CI 0·34-2.0); P= 0·82). Secondary
outcome variables for vitamin D deficient children were also comparable
between the two groups (data not shown).
Discussion
In this randomized, double blind, placebo controlled
trial, we observed that vitamin D (cholecalciferol, vitamin D 3)
administered in a single oral dose of 100,000 IU to children aged 6
months to 5 years with severe pneumonia (as defined by WHO: any child
with cough/fast breathing with lower chest indrawing), hastens the
resolution of lower chest indrawing by one hour which is statistically
significant but may not be relevant, clinically. There was no
significant reduction in the time taken for complete recovery from
pneumonia, duration of hospitalization, and fever clearance time.
Further, the supplementation did not prevent the recurrence of pneumonia
in next six months. Similar observations were made in a subgroup
analysis that included only vitamin D deficient children.
Lack of a wider therapeutic or preventive benefit of
vitamin D supplementation in severe pneumonia was observed despite a
significant improvement in serum 25(OH)D levels. Efficacy of vitamin D
supplementation for improving the vitamin D status was evident from a
significant reduction in proportion of vitamin D deficient children in
the supplementation group. Vitamin D supplementation did not bring about
any significant change in the serum levels of immunoglobulins IgA and
IgG of study participants. A marginal increase occurred in serum IgM
levels in children supplemented with vitamin D. Serum cathelicidin, an
antimicrobial peptide, said to be responsible for the immunity enhancing
property of vitamin D, remained unaffected after vitamin D
supplementation.
We were unable to replicate the "effect" seen in the
Kabul study [14] i.e., reducing the frequency of recurrence of
pneumonia by vitamin D supplementation. The difference can be explained
on the basis of certain parameters that were uneven between the two
studies including the definition of pneumonia. We included all cases
that satisfied WHO definition of severe pneumonia [18], including those
with wheeze; whereas the Kabul study excluded all children with wheeze.
More than 80% of our children had wheeze and could be either harboring a
viral infection or having a component of allergic respiratory disorder.
Studies have suggested that 1,25(OH)D may induce the production of
immune markers only in presence of some specific pathogens [27,28] and
probably those pathogens were in low numbers in our study. Secondly, the
illness severity was more in our children since all our participants had
severe pneumonia; Kabul study included children with both pneumonia and
severe pneumonia. There is a possibility that in more severe cases, the
conversion of 25(OH)D to 1,25(OH)D, which is the active metabolite, gets
hampered [29]. It would have been very appropriate to also measure
vitamin D status at 6 months, as 25OHD concentrations between the
control and active groups were nearly similar at 3 months and would most
probably have been no different at 6 months. This discussion does raise
the question about whether or not the response might have been different
if 100,000 IU had been given more frequently (eg. every three months).
We could not compare our results on early resolution of severe pneumonia
since the Kabul study was underpowered to comment on this outcome due to
less number of children with severe pneumonia.
Our study had certain limitations. We included a
heterogeneous group of conditions under the blanket diagnosis of severe
pneumonia, because of the pitfalls of its definition. WHO criteria to
identify severe pneumonia are highly sensitive but have a low
specificity. Clinically, most of these children had a wheezy illness and
very few had consolidation/bacterial pneumonia; a few may have been
suffering from allergic respiratory illness. Moreover, we did not
attempt a microbiological diagnosis of pneumonia by lung tap or
bronchoalveolar lavage. Immune and clinical response to vitamin D may
vary for bacterial/viral illness. While studying immune responses, we
did not have any outcome measures related to cell-mediated immunity.
Flow-cytometry and evaluation of cytokines could not be undertaken for
want of logistics. We admit that our workup for parameters of humoral
immunity was also not holistic. Finally, we were able to exclude
clinical rickets at enrolment but not severe vitamin D deficiency based
on 25OHD levels. No child developed clinical rickets during the course
of the study. An ethical issue may not arise as there is no clear
consensus about whether asymptomatic vitamin D deficiency needs to be
treated.
To conclude, we did not find any robust evidence to
recommend routine supplementation of vitamin D to children with severe
pneumonia to hasten the resolution of illness or to prevent recurrence
of further episodes in next 6 months. It however remains to be seen
whether similar results are obtained in children with radiological
pneumonia, and those belonging to other racial groups and nutritional
status.
Acknowledgments: The trial was supported
by funding from the Indian Council of Medical Research, New Delhi. The
drug and placebo used in the study were provided by Zuventus Healthcare
Ltd. India.
Contributors: The study was conceived by PG,
building on a previous study by PG and NB. PG, PD, DS, IRK, AKB, and SVM
contributed to study design and writing the proposal for research. Data
collection was handled by NS, and supervised by PD, DS, and PG. IRK and
SVM supervised the laboratory work-up of immune markers, and vitamin D
status, respectively. Statistical analysis was carried by AKB and PG.
Literature search was conducted by NB, NS, and PG. Initial draft of the
manuscript was written by NB and NS which was edited and refined by PG.
PD, DS, SVM, IKK, and AKB provided critical inputs to the draft
manuscript. The manuscript was seen and approved by all authors.
Funding: Indian Council of Medical Research.
Competing interests: Zuventus Healthcare Ltd.
India provided the drug/placebo and are manufacturers of vitamin D
formulations. They, however, had no role in study design, data
collection, data analysis, data interpretation, or writing of the
manuscript.
|
What is Already Known?
Observational studies have shown an association between vitamin
D deficiency and respiratory tract infections, probably due to
its immune-enhancing properties
What This Study Adds?
Vitamin D supplementation to children
with pneumonia may improve the vitamin D status and offer a
marginal benefit in reduction of disease severity and
recurrence. However the advantage offered is neither clinically
significant nor consistent to warrant routine supplementation of
vitamin D in children below five years of age with pneumonia.
|
References
1. World Health Organization. Pneumonia fact sheet N
331. From http://www.who.int/mediacentre/factsheets/fs331/en.
Accessed January 23, 2016.
2. McNally JD, Leis K, Matheson LA, Karuananyake C,
Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with
severe acute lower respiratory infection. Pediatr Pulmonol.
2009;44:981-8.
3. Roth DE, Shah R, Black RE, Baqui AH. Vitamin D
status and acute lower respiratory infection in early childhood in
Sylhet, Bangladesh. Acta Paediatr. 2010;99:389-93.
4. Karatekin G, Kaya A, Saliho Ιglu
O, Balci H, NuhoΙglu
A. Association of subclinical vitamin D deficiency in newborns with
acute lower respiratory infection and their mothers. Eur J Clin Nutr.
2009;63:473-7.
5. Ahmed P, Babaniyi IB, Yusuf KK, Dodd C, Langdon G,
Steinhoff M, Dawodu A. Vitamin D status and hospitali-zation for
childhood acute lower respiratory tract infections in Nigeria. Paediatr
Int Child Health. 2015; 35:151-6.
6. Shah N, Ramankutty V, Premila PG, Sathy N. Risk
factors for severe pneumonia in children in south Kerala: a
hospital-based case-control study. J Trop Pediatr. 1994; 40:201-6.
7. Banajeh SM. Nutritional rickets and vitamin D
deficiency-association with the outcomes of childhood very severe
pneumonia: A prospective cohort study. Pediatr Pulmonol.
2009;44:1207-15.
8. Wayse V, Yousafzai A, Mogale K, Filteau S.
Association of subclinical vitamin D deficiency with severe acute lower
respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563-7.
9. Liu PT, Stenger S, Li H, Wenzel L, Tan BH ,
Krutzik SR, et al. Toll-like receptor triggering of a vitamin
D-mediated human antimicrobial response. Science. 2006;311:1770-3.
10. Muhe L, Lulseged S, Mason KE, Simoes EAF. Case
control study of the role of nutritional rickets in the risk of
developing pneumonia in Ethiopian children. Lancet. 1997; 349:1801-4.
11. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada
Y, Ida H. Randomized trial of vitamin D supplementation to prevent
seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255-60.
12. Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach
I. Vitamin D supplementation in children may prevent asthma exacerbation
triggered by acute respiratory infection. J Allergy Clin Immunol.
2011;127:1294-6.
13. Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg
FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin D
supplementation and risk of acute respiratory infection in Mongolia.
Pediatrics. 2012;130:e561-7.
14. Manaseki-Holland S, Qader G, Isaq Masher M, Bruce
J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D
supplementation to children diagnosed with pneumonia in Kabul: a
randomized controlled trial. Trop Med Int Health. 2010;15:1148-55.
15. Manaseki-Holland S, Maroof Z, Bruce J, Mughal
MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of
pneumonia of vitamin D supplementation by quarterly bolus dose to
infants in Kabul: a randomized controlled superiority trial. Lancet.
2012;379:1419-27.
16. Choudhary N, Gupta P. Vitamin D supplementation
for severe pneumonia-a randomized controlled trial. Indian Pediatr.
2012;49:449-54.
17. Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D
for prevention of respiratory tract infections: A systematic review and
meta-analysis. J Pharmacol Pharmacother. 2012;3:300-3.
18. World Health Organization. Technical bases for
the WHO Recommendations on the Management of Pneumonia in Children at
First level Health Care. Report no. WHO/ARI/91·20. Geneva: WHO; 1995.
19. WHO Child and Adolescent Health and Development
(CAH). Integrated management of neonatal and childhood illness and HIV.
Physician Chart Booklet. New Delhi: Ministry of Health and Family
Welfare, Govt. of India; 2007.
20. World Health Organization. Physical status: The
Use and Interpretation of Anthropometry. Report of WHO Expert Committee.
Geneva: WHO; 1987.
21. World Health Organization Anthro for personal
computers, version 3·2·2, 2011: Software for assessing growth and
development of the worlds children. Geneva: WHO; 2010.
http://www.who.int/childgrowth/software/en/ (accessed September 2011).
22. World Health Organization, Multicentre Growth
Reference Study Group. WHO Child Growth Standards:
Length/height-for-age, weight-for-age, weight-for-length,
weight-for-height and body mass index-for-age: Methods and development.
Geneva: World Health Organization; 2006.
23. Agarwal R, Singh V, Yewale V. RTI Facts. Indian
Academy of Pediatrics Consensus Guidelines on Rational Management of
Respiratory Tract Infections in Children. Mumbai: Indian Academy of
Pediatrics; 2006.
24. Institute of Medicine Food and Nutrition Board.
Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC:
National Academy Press; 2011.
25. Nicholson JF, Pesce MA. Reference ranges for
laboratory tests and procedures. In: Behrman RE, Kliegman RM, editors.
Nelson Textbook of Pediatrics. 17th edition. Philadelphia: Saunders;
2003·P·2396-427.
26. Hollick MF. The role of vitamin D for bone health
and fracture prevention. Curr Osteoporos Rep. 2006;4:96-102.
27. Bhalla AK, Amento EP, Clemens TL, Holick MF,
Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3
in human peripheral blood mononuclear cells; presence in monocytes and
induction in T lymphocytes following activation. J Clin Endocrinol Metab.
1983;57:1308-10.
28. Provvedini DM, Deftos LJ, Manolagas SC.
1,25-dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic
changes in normal human monocytes consistent with their differentiation
into macrophages. Bone. 1986;7:23-8.
29. Pletz MW, Terkamp C, Schumacher U, Rohde G, Schόtte
H, Welte T, et al; CAPNETZ-Study Group. Vitamin D deficiency in
community acquired pneumonia: low levels of 1,25(OH)2 D are associated
with disease severity. Respir Res. 2014;15:53.
|
|
|
 |
|

