|
|
|
Indian Pediatr 2016;53: 1019-1021 |
 |
A Novel
Protein C Mutation Causing Neonatal Purpura Fulminans
|
|
Usha Devi R, Mangala Bharathi S and Nikesh Kawankar
From Department of Neonatology, Institute of child
health and hospital for children, Egmore, Chennai, India.
Correspondence to: Dr Mangala Bharathi S,
Department of Neonatology, Institute of child health and hospital for
children, Egmore, Chennai-600008, India.
Email:
[email protected]
Received: September 19, 2015;
Initial review: December 15, 2016;
Accepted: July 02, 2016.
|
Background: Neonatal purpura fulminans due to
congenital protein C deficiency is a rare disorder. Case
characteristics: A four-day-old neonate presented with
multiple necrotic skin lesions with abnormal coagulation profile.
Intervention and outcome: Skin lesions responded to repeated plasma
transfusions but the neonate developed bilateral retinal detachment. A
novel homozygous PROC gene mutation was noted in the neonate.
Message: Molecular diagnosis and prenatal counseling in neonatal
purpura fulminans are vital considering the poor outcome.
Keywords: Bleeding, Coagulation, Genetics, Neonate.
|
|
Purpura fulminans in neonates is a rapidly
progressive thrombotic disorder manifesting as hemorrhagic skin
infarction and disseminated intravascular coagulation. Congenital
protein C deficiency presenting as neonatal purpura fulminans is an
exceedingly rare condition with an incidence of 1 in 4 million [1]. It
is caused by homozygous or compound heterozygous mutations in PROC
gene with an autosomal recessive inheritance [2]. We report a case of
novel homozygous PROC gene transversion mutation.
Case Report
A four-day-old male neonate was noted to have
multiple necrotic skin lesions in thigh and sole of left leg on day 2 of
life. He was third in birth order and born out of a term uneventful
pregnancy to parents with second degree consanguinity. His vitals were
stable and he was feeding well since birth. A new ecchymotic patch
appeared over the entire right buttock on day 4. The lesions progressed
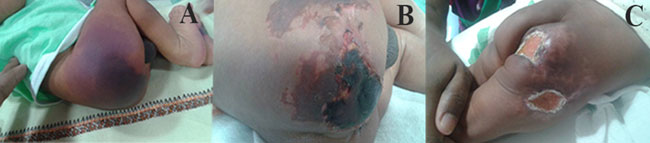
rapidly with irregular central hemorrhagic necrosis (Fig. 1)
surrounded by erythema. Investigation revealed thrombo-cytopenia
(platelets count 65×10 9/L),
prolonged prothrombin time (19.2 s INR-2) and activated partial
thromboplastin time (69 s) with normal hemoglobin and leucocyte counts.
He had grossly elevated levels of D-dimer (8590 ng/mL) and fibrin
degradation products (10 mcg/m; normal <5) suggestive of disseminated
intra-vascular coagulation (DIC). Doppler of the cranial and renal
vessels did not reveal any thrombosis. Computed tomogram of brain was
normal. Ophthalmological examination revealed bilateral leucocoria and
the B scan revealed bilateral vitreous hemorrhage and funnel shaped
complete retinal detachment.
 |
|
Fig.1 Multiple necrotic skin lesions
(various stages) of purpura fulminans in a neonate. (see color
images at website)
|
His elder female sibling had a similar clinical
presentation in the neonatal period with multiple cutaneous infarcts and
DIC. She did not respond to treatment with fresh frozen plasma (FFP)
transfusions and heparin therapy and died at one month of age.
Considering this, a detailed laboratory work up to exclude congenital
prothrombotic disorder was initiated. Protein C antigen (12.28%; normal
24-51) and activity (11%; normal 28-54) [3] estimated by chromogenic
assays were found to be markedly decreased. Protein S and anti-thrombin
III levels, were in the normal range. Parental protein C assay showed
normal maternal levels. Father had low normal levels of protein C
antigen (79%; normal 70-140) and decreased protein C activity (55%;
normal 70-130).
Genomic DNA was isolated from the peripheral blood of
the neonate and parents using Flexigene DNA extraction kit (Qiagen,
Germany). Nine polymerase chain reaction (PCR) procedures were carried
out for the 9 exons. The primers for exons 1, 2, 4/5, 7 and 8 were
designed according to Lind, et al. [4]. The amplicons were
sequenced using Big Dye Terminator cycle sequencing kit on an ABI 3130XL
genetic analyzer (Applied Biosystems).
The causative molecular defect was a novel
p.His253Tyr (C>T) transversion at the codon 253 of protein C gene. This
missense mutation in exon 8 at codon 253 His>Tyr predicted a protein
damaging serine protease domain to cause the protein C deficiency. This
mutation was present in a homozygous state in our symptomatic neonate
and in a heterozygous state in both asymptomatic parents (Web
Fig. 1).
This severe congenital deficiency of Protein C was
treated with multiple transfusions of FFP (20 mL/kg twice daily) along
with low molecular weight heparin (LMWH) during the first week. Protein
C concentrate was not available. He was started on oral anticoagulant
acenocoumaral from the second week. One week later, the estimated INR
was 4. LMW heparin was stopped and acenocoumaral dose was titrated to
attain target INR of 2.5-3.5. Skin lesions healed slowly over a month (Fig.
1) requiring FFP transfusions till the third week. Other deranged
parameters during admission also normalized at discharge (platelet count
450 × 109/L, D-dimer-490 ng/mL,
FDP-2 mcg/mL). The child is on follow up with long term anticoagulant
therapy. No breakthrough thrombotic events were noted till now.
Discussion
Protein C is a vitamin K dependant anticoagulant that
regulates thrombosis. Its role is especially important in the slow
flowing venous circulation where there is prolonged exposure of
procoagulant proteins and platelet phospholipids to the vessel wall with
high risk of venous thrombosis. Neonatal purpura fulminans is a severe
manifestation of homozygous Protein C deficiency usually presenting
within few hours of birth with cutaneous infarcts and features of DIC.
Thrombosis of cerebral vasculature and ophthalmologic complications
including vitreous hemorrhage and retinal detachment resulting in
partial or complete blindness are well known, with onset reported even
in fetal period [5], and often not amenable to correction by postnatal
management.
More than 230 unique mutations in PROC gene
have been identified as on date [2] . Most of them are missense/nonsense
mutations. Severe protein C deficiency often remains underdiagnosed as
parents are asymptomatic and newborns have physiologically low levels of
protein C along with difficulties in interpretation during the acute
phase. It is possible that many of them die during the phase of DIC even
before a diagnosis can be made.
Neonatal purpura fulminans is a medical emergency
that warrants rapid normalization of plasma Protein C activity. One
ml/kg of FFP may increase plasma protein C concentration by only 1 IU/dL
[6]. Highly purified protein C concentrate (Ceprotin) is an alternative
to repeated FFP transfusions [7]. After the acute phase, patients have
to be transitioned to long term anticoagulation therapy. Breakthrough
thrombotic events despite anticoagulation warrant exogenous protein C
administration. Liver transplantation from living donors have been
performed occasionally resulting in permanent cure [8,9].
Molecular diagnosis gives the parents an option of
reliable prenatal diagnosis in the next pregnancy. This is crucial
considering the poor prognosis and the need for multidisciplinary care
to address the ophthalmological and neurological sequelae of this
dreaded condition.
Contributors: UDR, MBS: managed the
patient; UDR: reviewed the literature and drafted the initial version of
the manuscript; MBS: contributed to literature review and critically
revised the manuscript; NK: did the DNA sequencing and identified the
molecular defect. All the authors contributed to drafting of the
manuscript and approved the final version of the manuscript.
Funding: None; Competing interests:
None stated.
References
1. Goldenberg NA, Manco-Johnson MJ. Protein C
deficiency Haemophilia. 2008;14:1214-21.
2. Knoebl PN. Severe congenital protein C deficiency:
the use of protein C concentrates (human) as replacement therapy for
life-threatening blood-clotting complications. Biol Targets Ther.
2008;2:285-96.
3. Price VE, Ledingham DL, Krümpel A, Chan AK.
Diagnosis and management of neonatal purpura fulminans. Semin Fetal
Neonatal Med. 2011;16:318-22.
4. Lind B, van Solinge WW, Schwartz M, Thorsen S.
Splice site mutation in the human protein C gene associated with venous
thrombosis: demonstration of exon skipping by ectopic transcript
analysis. Blood. 1993;82:2423-32.
5. Kirkinen P, Salonvaara M, Nikolajev K, Vanninen R,
Heinonen K. Antepartum findings in fetal protein C deficiency. Prenat
Diagn. 2000;20:746-9.
6. Marlar RA, Montgomery RR, Broekmans AW. Diagnosis
and treatment of homozygous protein C deficiency. Report of the Working
Party on Homozygous Protein C Deficiency of the Subcommittee on Protein
C and Protein S, International Committee on Thrombosis and Haemostasis.
J Pediatr. 1989;114:528-34.
7. de Kort EHM, Vrancken SLAG, van Heijst AFJ,
Binkhorst M, Cuppen MPJM, Brons PPT. Long-term subcutaneous protein C
replacement in neonatal severe protein C deficiency. Pediatrics.
2011;127: e1338-42.
8. Lee MJ, Kim KM, Kim JS, Kim YJ, Lee YJ, Ghim TT.
Long-term survival of a child with homozygous protein C deficiency
successfully treated with living donor liver transplantation. Pediatr
Transplant. 2009;13:251-4.
9. Monagle K, Ignjatovic V, Hardikar W, Newall F,
Monagle P. Long-term follow-up of homozygote protein C deficiency after
multimodal therapy. J Pediatr Hematol Oncol. 2014;36:e452-5.
|
|
|
 |
|

