|
|
|
Indian Pediatr 2015;52:
984-986 |
 |
Alveolar Capillary Dysplasia as a Cause of
Persistent Pulmonary Hypertension
|
|
Abdul Razak, Pankaj Kumar Mohanty and Karthik Nagesh
N
From Department of Neonatology, Manipal Hospital.
Bangalore, India.
Correspondence to: Dr N Karthik Nagesh, Head,
Department of Neonatology, Manipal Group of Hospitals,
Bangalore, India.
Email:
[email protected]
Received: April 07, 2015;
Initial review: May 20, 2015;
Accepted: August 21, 2015.
|
|
Background: Persistent pulmonary
hypertension (PPHN) in a term or late preterm has varied etiology.
Case characteristics: A late preterm neonate operated for esophageal
atresia with tracheo-esophageal fistula was complicated by severe
pulmonary hypertension and unable to be weaned off from respiratory
support. Outcome: The neonate expired by 15 weeks of life;
diagnosis was made on postmortem lung biopsy. Message:
Alveolarcapillary dysplasia should be considered in a neonate with
idiopathic refractory PPHN, if associated with anomalies.
Keywords: Alveolar capillary dysplasia,
Esophageal atresia, Late preterm.
|
|
Persistent pulmonary hypertension (PPHN) in the
neonates is commonly seen with parenchymal lung diseases (pneumonia,
meconium aspiration syndrome) and congenital cardiac diseases. When
these are not the underlying cause of pulmonary hypertension, and if it
is refractory to usual medical therapies, it presents a diagnostic
challenge. We report a late preterm neonate operated for esophageal
atresia with severe persistent pulmonary hypertension where the
diagnosis was made on post-mortem lung biopsy.
Case Report
A 36-weeks gestational age (2560g weight), male, was
born to a primigravide mother via emergency LSCS in view of
polyhydramnios and preterm prolonged rupture of membranes. The infant
was born non-vigorous through meconium stained liquor and required
tracheal suctioning with positive pressure ventilation for 30 secs.
After stabilization, the infant was transferred to neonatal intensive
care unit with high flow oxygen support. The antenatal scans showed
evidence of esophageal atresia with trachea-esophageal fistula. Surgical
repair was performed after the initial stabilization and the infant was
ventilated preoperatively. Respiratory deterioration after 12 hrs
necessitated switch to high frequency ventilation from conventional
ventilation. Blood gases showed severe respiratory acidosis, X-ray
was unremarkable and echocardiogram showed elevated pulmonary pressures.
Apart from PPHN, the deterioration was also thought due to chemical
pneumonitis, as there was an anastomotic leak visible on dye study
performed on 5 th
post-operative day; the leak was managed conservatively. The infant
remained ventilator-dependent, and the oxygenation indices (were
persistently high, 20). There was severe gastro-esophageal reflux
(diagnosed by barium meal) and Ventilator-associated pneumonia, which
led to repeated extubation failures. Endotracheal cultures grew
Acinetobacter and Pseudomonas, which were treated with appropriate
antibiotics. Feeding jejunostomy was performed at 8 weeks of postnatal
life in view of severe gastro-esophageal reflux. Echocardiogram perfomed
initially showed persistent pulmonary hypertension with large Ductus
arteriosus (bidirectional shunt with right to left predominance) and
partial anomalous pulmonary venous connection (PAPVC). PPHN was managed
with nitric oxide, sildenafil, milrinone and bosentan, with marginal
improvement. CT angiography was done which confirmed three pulmonary
vein opening to left atrium and one vein was stenotic which opened to
SVC. The repeat serial echocardiograms showed persistent large ductus
arteriosus with shunting now predominantly from left to right and hence
a decision to ligate the duct was made. Surgical ligation of
hemodynamically-significant ductus arteriosus was performed at 11 weeks
of postnatal life. The infant remained ventilator-dependent with high
requirements despite feeding jejunostomy and surgical ligation of duct.
Clinical discussions favoured primary PPHN and severe Chronic lung
disease due to various multiple hits and the same was counselled to
parents. Simultaneously, attempts to rule out the primary lung pathology
for ventilator dependency were carried further.
Cystic fibrosis screening was negative including the
common mutation analysis. High resolution chest CT was done to rule out
other possibilities like idiopathic pulmonary fibrosis, pulmonary
lymphagiectasia and structural malformations; it showed bilateral lower
lobe collapse, hyperinflated upper lung fields with mild septal
prominence and a small airspace cavity in the right paracardiac area,
signs of bronchopulmonary dysplasia. Baby remained critically ill with
extensive intensive care support, eventually developed
cardio-respiratory failure and died on day 103 of life.
Histopathological examination of lung was performed after consent from
parents; it revealed features consistent with alveolar capillary
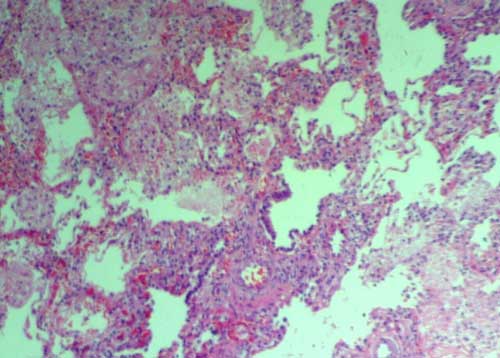
dysplasia (Fig. 1).
 |
|
Fig. 1 Misaligned pulmonary veins,
scarce dilated pulmonary capillaries located away from alveolar
epithelium, absent alveolo-capillary barrier and museularisation
of distal arterioles. (See color image at website).
|
Discussion
Alveolar capillary dysplasia with misalignment of the
pulmonary veins (ACD/MPV) is a rare and universally fatal disorder
leading to respiratory failure early in life [1]. Most of affected
infants present shortly after birth or within 48 hrs of life with
hypoxic respiratory failure and are refractory to all medical therapies
[2]. Newborns affected with ACD/MPV present with minimal or no
parenchymal lung disease and severe idiopathic PPHN. Profound hypoxemia,
with partial pressures of oxygen in arterial blood less than 30 mm Hg,
and severe metabolic acidosis consistent with pulmonary hypertensive
crisis and right ventricular failure are nearly always present. ACD/MPV
should be suspected in neonates with primary/idiopathic PPHN who fail to
respond to pulmonary vasodilator therapy including nitric oxide therapy
and ECMO. While most of the affected babies present in neonatal period
and expire early; so far four reports have been published of infants
presenting with fulminant disease later (5 week to 7 month age) [3-6].
Two of them presented with symptoms serious enough to warrant brief
periods of respiratory observation in a neonatal intensive care unit
before discharge [3,6], the remaining two appeared asymptomatic and were
discharged home as well babies [4,5]. Survival beyond three months after
the onset is rare. Diagnosis of ACD/MPV is made only on histological
grounds, characterized by misaligned pulmonary veins, scarce dilated
pulmonary capillaries located away from alveolar epithelium, absent
alveolo-capillary barrier, and muscularization of distal arterioles.
Newborns with ACD/MPV have multiple other congenital
malformations of gastrointestinal tract, cardiovascular system, and
genitourinary system in almost 80% of cases. PAPVC with esophageal
atresia and tracheoesophageal fistula in our case with progressive
pulmonary insufficiency prompted us to perform lung biopsy to rule out
ACD/MPV. Antemortem lung biopsy was offered to parents; however it was
not performed because of unstable clinical condition. Early diagnosis of
this condition would prevent the newborn with ACD/MPV to undergo
invasive therapies. However, CT scan performed showed dilated central
pulmonary arteries; this finding was not given much importance and could
have helped us in early diagnosis [7].
About 10% of alveolar capillary dysplasia are
reported to have familial association [8]. Candidate gene involvement is
now being researched and some case reports showed haplo-insufficincy for
the fork-head FOX transcription factor gene cluster which may
present in 40% of cases [9]. Genetic testing was not performed in our
case.
The clinical approach to newborns with ACD/MPV is on
the same lines as that for persistent primary pulmonary hypertension
[10]. Index of suspicion should be high if a neonate with idiopathic
PPHN is a non-responder to usual medical therapies and especially if
there are any associated anomalies of the gastrointestinal,
genitourinary, and cardiovascular systems. Early diagnosis by histologic
evaluation of antemortem lung biopsy in these neonates warrants against
unnecessary therapies and prolongation of medical treatment.
Contributors: All authors were involved in
patient-management, and contributed to the review of literature. All
authors approved the final version of the manuscript.
Funding: None; Competing interests: None
stated.
References
1. Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar
capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172-9.
2. Janney CG, Askin FB, Kuhn C. Congenital alveolar
capillary dysplasia–an unusual cause of respiratory distress in the
newborn. Am J Clin Pathol. 1981;76:722-7.
3. Ahmed S, Ackerman V, Faught P, Langston C.
Profound hypoxemia and pulmonary hypertension in a 7-month-old infant:
late presentation of alveolar capillary dysplasia. Pediatr Crit Care
Med. 2008;9:e43-e6.
4. Abdallah HI, Karmazin N, Marks LA. Late
presentation of misalignment of lung vessels with alveolar capillary
dysplasia. Crit Care Med. 1993;21:628-30.
5. Shankar V, Haque A, Johnson J, Pietsch J. Late
presentation of alveolar capillary dysplasia in an infant. Pediatr Crit
Care Med. 2006;7:177-9.
6. Shehata BM, Abramowsky CR. Alveolar capillary
dysplasia in an infant with trisomy 21. Pediatr Dev Pathol.
2005;8:696-700.
7. Marshall GB, Silva CI, English JC, Levy RD, Müller
NL. Misplaced pulmonary arteries in an adult patient with pulmonary
hypertension. Br J Radiol. 2010;83:e5-9.
8. Vassal H, Malone M, Petros A, Winter R. Familial
persistent pulmonary hypertension of the newborn resulting from
misalignment of the pulmonary vessels (congenital alveolar capillary
dysplasia). J Med Genet. 1998;35:58-60.
9. Yu S, Shao L, Kilbride H, Zwick D.
Haploinsufficiency of FOXF1 and FOXC2 genes associated
with lethal alveolar capillary dysplasia and congenital heart disease.
Am J Med Genet. 2010;152A:1257-62.
10. Miranda J, Rocha G, Soares H, Vilan A, Brandao O,
Guimarães H. Alveolar capillary dysplasia with misalignment of pulmonary
veins (ACD/MPV): A case series. Case Rep Crit Care. 2013;2013:1-4 .
|
|
|
 |
|

