|
|
|
Indian Pediatr 2015;52:
965-972 |
 |
Mortality and Other
Outcomes in Relation to First Hour Fluid Resuscitation Rate:
A Systematic Review
|
|
A Tripathi, SK Kabra, *HPS Sachdev and R Lodha
From Departments of Pediatrics; AIIMS, and *Sitaram
Bhartia Institute of Science and Research; New Delhi, India.
Correspondence to: Dr Rakesh Lodha, Department of
Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New
Delhi 110 029, India.
Email:
[email protected]
Received; April 04, 2015;
Initial review: May 29, 2015;
Accepted: July 29, 2015.
|
Objective: To
determine the effect of different regimen of first hour fluid
administration rates on mortality and severe consequences of impaired
circulation in 2 to 60 months old children with impaired circulation.
Design: Systematic review of randomized
controlled trials.
Data sources: Various databases including PubMed,
Cochrane Library and EMBASE were searched.
Results: We found only two relevant
trials; one was excluded as there was no comparator arm. Only one study
(The FEAST Trial) compared boluses with maintenance fluid alone in
children with severe febrile illness and one or more signs of impaired
perfusion. The 48-hour mortality was more in the bolus group (RR 1.45,
95% CI 1.13,1.86). The quality of evidence is rated as ‘moderate’. For
the children who met the WHO criteria for shock (severely impaired
circulation) (n=65 children), those receiving boluses had higher
mortality (RR 2.40, 95% CI 0.84, 6.88); the quality of evidence was
rated as ‘very low’.
Conclusions: A single large randomized controlled
trial conducted in low-resource settings indicates that administration
of fluid bolus is associated with higher mortality in comparison to the
maintenance fluids alone in children with severe febrile illness and one
or more signs of impaired perfusion. The findings are not generalizable
to contexts with different severity of and different causes of shock and
in centers with better facilities. There is urgent need for research in
different settings to determine the optimal rate of fluid resuscitation
in the first hour in children presenting with impaired circulation,
particularly with severely impaired circulation.
Keywords: Infection, Intravenous fluids, Septic shock.
|
|
S
epsis and septic shock are important causes of
morbidity and mortality in children in developing countries [1]. The
mortality rate in children with septic shock may be as high as 50% [2].
The outcome is worse when shock is associated with co-morbidities
and organ dysfunction [3]. The major physiological abnormality in shock
is hypovolemia, and early repletion by appropriate fluid infusion should
improve the physiology and survival. Fluid boluses include rapid
administration of crystalloids or colloids. The outcome may be largely
dependent on the quantity of fluids used in the first hour [4].
Pediatric life support training program recommends administration of up
to 60 ml/kg of fluids in the first hour, preferably within the first 15
minutes of diagnosing shock [5]. World Health Organization (WHO)
advocates exercising caution in liberal fluid administration policy,
especially in children with advanced shock in resource-limited
conditions [6]. There may be differences in response in children
with severe malnutrition, age <60 months, severe anemia, severe
dehydration and varying severity of impaired circulation.
Evidence from randomized controlled trials (RCTs) is
lacking to support all components of fluid resuscitation guidelines
[5,7]. Moreover, these guidelines have been developed in high-resource
countries with well-developed intensive care services where
malnutrition, particularly the severe form, is uncommon. A large trial
(The FEAST trial) [8] in a resource-limited setting has questioned the
use of boluses in children with severe febrile illness and impaired
perfusion. It is, therefore, important to systematically evaluate the
available evidence to determine the appropriate fluid adminis-tration
strategy in children with impaired circulation. The aim of this
systematic review was to evaluate the effect of first hour
fluid-administration rates on mortality and other outcomes in 2-to
60-month old children with impaired circulation. The primary objective
was to determine the effect of first hour fluid administration rates on
mortality and severe consequences of impaired circulation in children.
Secondary objective was to determine the effect of first hour
fluid-administration rates on improved circulation.
Methods
Criteria for Selecting Studies
We restricted our review to controlled clinical
trials (randomized or quasi-randomized) in 2- to 60-month old children
that compared the different rates of isotonic fluid administration in
the first hour of shock/impaired circulation (defined as the presence of
one or more of the following signs: systolic blood pressure less than
the age appropriate cut-off, cold peripheries, capillary refill time >2
s). There was no language restriction. Studies on children suffering
from burns, hemorrhage, anaphylaxis and cardiac disorders were excluded.
We categorized fluid regimens as follows:
• Standard care: Isotonic fluid
intravenous (IV) boluses or rapid continuous infusions of 20-60
ml/kg in addition to maintenance rates within the first hour of
resuscitation (control group).
• Maintenance fluids only: Isotonic
fluid IV at maintenance rates only in the first hour of
resuscitation.
• Small bolus plus maintenance:
Isotonic fluid boluses or rapid continuous infusions of a maximum of
20 mL/kg in addition to maintenance rates within the first hour of
resuscitation.
Outcome Measures
Primary outcome measures were mortality in first week
and severe consequences of impaired circulation in the form of cardiac
failure, renal failure or neurological deterioration as defined by the
author. Secondary outcome measures were improvement in circulation
(responders) based on blood pressure (BP), pulse rate (PR) and capillary
refill at or before 6 hours, improvement in circulation based on BP, PR
and capillary refill at 24 hours and requirement of endotracheal
intubation and mechanical ventilation (indications as defined by the
investigators).
Data Sources and Search Strategy
We searched the Cochrane Central Register of
Controlled Trials, Pub Med (1966 to September 2014), EMBASE (1980 to
September 2014) by using appropriate terms. The search strategy is shown
in Web Appendix 1. Abstracts of all articles were read by
two authors independently and the relevant articles were selected. Full
text articles of selected studies were obtained. The references of the
selected articles were screened to identify any further eligible
studies. Disagreements were resolved by discussion.
Data Syntheses
We developed a structured data extraction form to
collect the relevant information from the selected papers. Data of
baseline characteristics, and primary and secondary outcome measures
were extracted in the pretested form by two authors independently.
Differences in the data were resolved by discussion. Co-interventions
were also documented. We had planned to perform statistical analysis
using the Review Manager software but due to paucity of relevant
studies, we provided the narrative synthesis instead of meta-analysis in
this review. The data were also synthesised using a ’Summary of
findings’ table. Risk ratio (RR) estimations with 95% confidence
intervals (CI) were used for binary outcomes [9].
Assessment of Risk of Bias
Two authors independently assessed the risk of bias
for each controlled trial using the criteria outlined in the ‘Cochrane
Handbook for Systematic Reviews of Interventions’ and those
recommended by Effective Practice and Organisation of Care (EPOC)
[10-14]. The judgment for each entry involved assessing the risk of bias
as ‘low risk’, ‘high risk’ or ‘unclear risk’. Any disagreements were
resolved by mutual discussion.
Assessment of Quality of Evidence
The quality of evidence for each of the efficacy and
safety outcomes was assessed using the Grading of Recommendations,
Assessment, Development and Evaluation (GRADE) approach. Key quality
elements assessed by GRADE included: risk of bias, precision,
consistency, directness of evidence and publication bias. The grade
evidence profiles were prepared by one reviewer and verified
independently by two reviewers.
Results
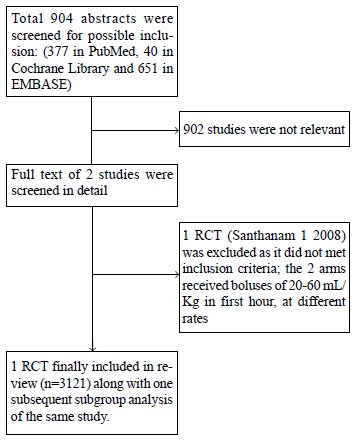
We could identify only two relevant RCTs, which
compared different rates of fluid administration in the first hour in
children presenting with impaired circulation (Fig. 1).
One trial had to be excluded as there was no comparator arm of ‘no
maintenance fluids’ [15]. Only one study - Fluid Expansion as
Supportive Therapy (FEAST Trial) [8] compared bolus with maintenance
fluid alone. We also identified articles reporting the subgroup analysis
of the data from the FEAST trial [16,17].
 |
|
Fig.1 The PRISMA flow chart.
|
Characteristics of Excluded Study
One trial was excluded as there was no comparator arm
[15]. They compared two rates of boluses (20-60 mL/kg within one
hour of admission).
Characteristics of Included Study
The summary of the selected study is shown in
Table I. In this RCT from Africa, in Stratum A, 3141 children
were randomized to a fluid bolus (20-40 mL/kg 5% albumin or normal
saline over 1 h) or maintenance fluids (2.5-4.0 mL/kg/h)
[8]. The median (IQR) age of participants was 24
(13,38) months; 62% had prostration, 15% were comatose and 83% had
respiratory distress. The majority (52%) of children had more than one
feature of impaired perfusion, most commonly severe tachycardia and cold
extremities. Moderate to severe acidosis was present in 51% of the
children and severe lactic acidosis (lactate
³5 mmol/L) in 39% of
the children. The mean (SD) hemoglobin level was 7.1(3.2) g/dL and the
glucose was 6.9 (3.9) mmol/l. Malaria was confirmed in 57% of the
children and 4% were positive for HIV infection. Only 17 (0.5%) children
were lost to follow-up for the primary end point. The median volume of
fluid administered was 20 mL/kg in the first hour and 40 mL/kg in the
first 8 h for both bolus-fluid groups, compared to 1.2 mL/kg and 10 mL/kg
at 1 and 8 h, respectively, in the no-bolus group [8,16].
TABLE I Summary of the Included Study (FEAST Trial)
|
Study |
Maitland, et al., 2011 [8] |
|
Study group |
Children were eligible for inclusion in the study if they were
between 60 d and 12 y of age and presented with a severe febrile
illness and with impaired perfusion. Major exclusion criteria
included: |
|
• Severe acute malnutrition |
|
• Gastroenteritis |
|
• Conditions where intravascular volume expansion is
contraindicated, viz., chronic renal failure, pulmonary edema |
|
• Non-infectious causes of severe illness: trauma; burns;
intoxication |
|
• Children who have already received volume expansion using an
isotonic volume expander during the current illness. |
|
Study setting |
Resource-limited settings in Sub Saharan countries— Kenya,
Tanzania and Uganda. |
|
Study type |
Two-strata (Stratum A and Stratum B), multicenter, open,
randomized, controlled study in six clinical sub-centers in
Kenya (one center), Tanzania (one center), and Uganda (four
centers). |
|
Selection criteria |
Children with severe febrile illness and clinical evidence of
impaired perfusion, Severe illness and impaired perfusion were
defined as follows: |
|
Severe febrile illness: one or more of the following: |
|
• Impaired consciousness: prostration or coma |
|
• Respiratory distress |
|
• Impaired perfusion: one or more of the following: |
|
• Capillary refill > 2s |
|
• Lower limb temperature gradient |
|
• Weak radial pulse volume |
|
• Severe tachycardia |
|
*Hypotensive shock (Allocated to Stratum B only): Systolic BP
<50, <60, <70 mm Hg for ages < 1y, 1-4y, >=5 y. |
|
Intervention |
Maintenance fluids only: Isotonic fluid IV at maintenance rates
in the first hour of resuscitation, with no additional IV
boluses or rapid continuous infusions. |
|
Standard care: Isotonic fluid IV boluses or rapid continuous
infusions of 20-60 ml/kg, in addition to maintenance rates,
within the first hour of resuscitation. An equal number of
children were randomized to receive one of the 2 types of fluids
as boluses: normal saline or 5% albumin. |
|
Outcomes |
Primary Endpoint: In-hospital mortality at 48 hours after
randomization. |
|
Secondary Endpoints: Mortality at 4 weeks, neurological sequelae
at 4 weeks and 24 weeks, episodes of hypotensive shock within 48
hours of randomization, adverse events related to fluid
resuscitation (pulmonary edema, intracranial hypertension or
severe allergic reaction to those receiving albumin). |
Risk of Bias in the Included Study
In the included study, the overall risk for bias was
assessed as ‘Low Risk based on the criteria of allocation
sequence concealment (selection bias), blinding of participants and
personnel (performance bias), blinding of outcome assessment (detection
bias), incomplete outcome data (attrition bias), selective outcome
reporting (reporting bias), comparability of baseline outcome and
characteristics, protection from contamination and other potential
sources of bias [8]. The details of risk of bias assessment are
mentioned in Web Appendix 2.
Effects of Intervention on Outcomes
Summary of outcome measures in the included study is
shown in Table II. Compared to the maintenance fluids, the
fluid bolus was associated with increased 48 h mortality (RR 1.45; 95%
CI 1.13,1.86) and increased mortality at 4 weeks (RR 1.39; 95% CI
1.11,1.74). There was no evidence that albumin performed in a different
manner than saline (albumin vs. saline bolus (RR; 1.0; 95% CI,
0.78,1.29) [8,16]. There was no evidence of difference between the two
groups in the risk of neurological sequelae at 4 weeks (RR 1.03; 95% CI
0.61,1.75) or the combined outcome of pulmonary edema or increased
intracranial pressure (RR 1.46; 95% CI 0.85, 2.53) [9,17]. In a
subsequent analysis, the effect of boluses on 48-hour all-cause
mortality was assessed in different sub-groups created by clinical
presentation at enrolment, hemodynamic changes over the first hour and
on different modes of death according to terminal clinical events (TCE)
[16].
TABLE II Summary of Outcome Measures in the
Included Study
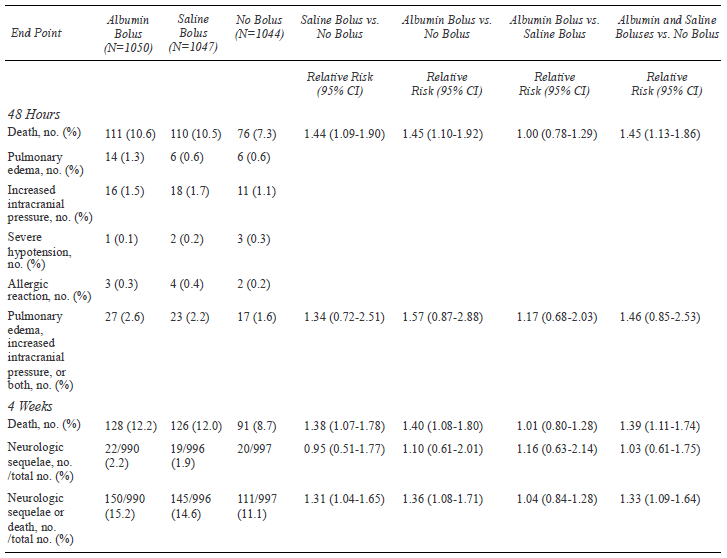
By one hour, shock had resolved (responders) more
frequently in bolus vs. control groups (43% vs.32%, P<0.001)
but excess mortality with boluses was evident in responders (RR 1.98;
95% CI 0.94,4.17; P=0.06) and ‘non-responders’ (RR 1.67; 95% CI
1.23, 2.28; P=0.001) [8]. The adverse effect of fluid boluses on
mortality was reported to be similar across various subgroups reported.
The difference was not found to be significant while comparing albumin
vs. saline bolus groups.
We graded the overall quality of evidence as
‘moderate’ as we downgraded the quality one notch in view of
indirectness (generalizability is limited as study was carried out in a
resource-limited setting where facilities for respiratory support were
not available; and majority of children had malaria. (Table
III).
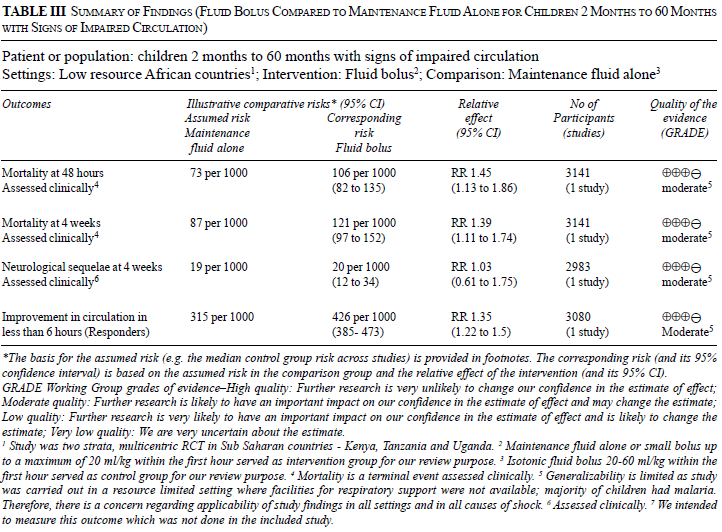
The authors presented the data for the main outcome
(mortality at 48 hours) in the subgroup of 65 children fulilling the WHO
definition of severely impaired circulation [8, 16, 17]. The mortality
was 48% and 20% in the two arms, respectively (RR 2.40; 95% CI 0.84,
6.88). The quality of evidence was downgraded as ‘very low’ based
on concerns of indirectness (as above), bias (as children were not
randomized based on the presence of severely impaired circulation; the
analysis is post-hoc; the numbers in the bolus and no-bolus arms are not
balanced — the ratio is >3:1 against an expected ratio of 2:1), and
imprecision (the confidence interval for the relative risk is wide and
includes 1) (Table IV).
Discussion
This systematic review could identify only one RCT
that met the inclusion criteria. This highlights the paucity of evidence
for formulation of guidelines for management of children with shock.
Data from this single study enrolling more than 3000 children with
severe febrile illness and impaired perfusion suggested significantly
higher mortality in bolus group compared to control arm in children
having one or more signs of impaired circulation. The higher mortality
rate in the bolus arm was consistent across all subgroups.
These findings are contrary to the current practice
recommendations for the management of shock [5,18-20]. The use of
intravenous fluids in management of shock has evolved over last two
centuries. Most of the information on fluid resuscitation in
children has been from observational studies (Web Appendix
3). Various professional bodies have recommended use of rapid fluid
boluses in children with shock [18-20]. Oliveira, et al.
[21] in 2008 highlighted the role of early goal-directed-therapy in
pediatric septic shock. In the 2012 Surviving Sepsis Campaign
guidelines, the recommendations for fluid resuscitation were restricted
to the industrialized world [20].
Though our findings are comparable to two earlier
reviews [22,23], there are concerns regarding the definition of impaired
circulation/ shock in the included study. The inclusion criteria were
broader than most shock-classification systems [24], and these results
refer to impaired perfusion rather than decompensated shock. While using
any one of the signs of shock increases the sensitivity, specificity
will be reduced significantly [24], and this may have led to inclusion
of children who did not have shock. There is lack of studies evaluating
different definitions of shock so as to determine the optimal
definition. The FEAST investigators had previously shown
poor-to-moderate inter-observer agreement for these signs used to
identify impaired perfusion [25]. It is important to note that the cause
of excess deaths in the FEAST trial was primarily refractory shock and
not fluid overload [16]. This indicates potential role of reperfusion
injury [16]. However, the current standard of care in intensive care
setting for management of impaired circulation is use of fluid boluses.
In these settings, the availability of invasive monitoring, mechanical
ventilation and vasoactive drug infusions may contribute to improved
outcomes.
Several concerns have been raised regarding
applicability or results of FEAST trial [26-30]. There is also concern
about impaired free-water excretion during severe infections [31]. It is
likely that the many of conditions that the FEAST study subjects had may
be adversely affected by extra intravenous volume infusion because of
high circulating levels of anti-diuretic hormone
[24,32,33]. There is also some concern regarding
the discrepancy in the inclusion criteria in the study protocol and the
published paper. The original study protocol does not mention severe
febrile illness as an inclusion criterion but the amended protocol (June
2011) mentions severe febrile illnesses as the inclusion criterion.
While the FEAST trial was conducted in a resource-
limited setting, further trials to determine the optimal fluid infusion
rate in children with shock should collect information on baseline
severity of illness using the PRISM or PIM scores, etiology of shock and
regarding the cardiac function as these were not available in the FEAST
study.
A single high-quality randomized controlled trial,
conducted in Africa in low-resource settings with no facilities for
mechanical ventilation, indicates that administration of fluid bolus
increases mortality in comparison to only maintenance fluids in 2- to
60-months old children with severe febrile illness and one or more signs
of impaired circulation. The overall quality of evidence was assessed as
‘moderate’. For the subgroup of children fulfilling the WHO-ETAT
criteria for severely impaired circulation, there was a trend towards
increased mortality in the bolus arm; however, the quality of evidence
was rated as ‘very low’. There is need for research in formulating
uniform diagnostic criteria for shock, identifying determinants of fluid
responsiveness, and further studies in different settings to determine
the optimal rate of fluid resuscitation in the first hour in children
presenting with signs of impaired circulation, particularly in intensive
care settings with optimal resources.
Acknowledgements: Dr Bipin Jose for assisting the
development of protocol and literature search. The experts participating
in the WHO Guideline Meeting — Paediatric Emergency Triage, Assessment
and Treatment at Geneva, Switzerland, 30 September - 2 October 2014 for
their comments.
Contributors: RL, SKK, HPS: conceptualized
the review, developed search strategy, developed the protocol, analyzed
the data and wrote the review, AT performed literature search, wrote the
manuscript. All authors approved the final version.
Funding: None; Competing interests; None
stated.
References
1. Bhutta ZA, Black RE. Global maternal, newborn, and
child health – so near and yet so far. N Engl J Med. 2013;369: 2226-35.
2. Sarthi M, Lodha R, Vivekanandhan S, Arora NK.
Adrenal status in children with septic shock using low-dose stimulation
test. Pediatr Crit Care Med. 2007;8:23-8.
3. Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier
M. Epidemiology of sepsis and multiple organ dysfunction syndrome in
children. Chest. 1996;109:1033-7.
4. Finfer S, Bellomo R, Boyce N, French J, Myburgh J,
Norton R, et al. A comparison of albumin and saline for fluid
resuscitation in the intensive care unit. N Engl J Med.
2004;350:2247-56.
5. Brierley J, Carcillo JA, Choong K, Cornell T,
Decaen A, Deymann A, et al. Clinical practice parameters for
hemodynamic support of pediatric and neonatal septic shock: 2007 update
from the American College of Critical Care Medicine. Crit Care Med.
2009;37:666-88.
6. WHO Pocket Book of Hospital Care for Children:
Guidelines for the Management of Common Illnesses with Limited
Resources. Available from:
http://www.asha.org/Members/ebp/compendium/guidelines/WHO-Pocket-Book-of-Hospital-Care-for-Children–Guidelines-for-the-Management-of-Common-Illnesses-with-Limited-Resources.htm
7. Brilli RJ, Goldstein B. Pediatric sepsis
definitions: Past, present, and future. Pediatr Crit Care Med.
2005;6:S6-8.
8. Maitland K, Kiguli S, Opoka RO, Engoru C,
Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in
African children with severe infection. N Engl J Med. 2011;364:2483-95.
9. Higgins JPT GS. Selecting studies and collecting
data. Cochrane Handbook for Systematic reviews of interventions. West
Sussex, England: John Wiley & Sons Ltd; 2008. p. 151-86.
10. Green S HJ. Cochrane Handbook for Systematic
Reviews of Interventions Version 5.1.0. The Cochrane Collaboration.
2011.
11. Schünemann HJ, Oxman AD, Vist GE, Higgins JPT,
Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and
drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane
Handbook for Systematic Reviews of Interventions [Internet]. Version
5.1.0. The Cochrane Collaboration, 2011. Available from:
www.cochrane-handbook.org. Accessed April 01, 2015.
12. Sterne JAC, Smith GD. Investigating and dealing
with publication and other biases. In: Smith GD, editor.
Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ
Books; 2001. p. 189-208.
13. Sterne JAC, Harbord RM. Funnel plots in
meta-analysis. Stata J. 2004;4:127-41.
14. Sterne JAC, Bradburn MJ, Egger M. Meta-analysis
in STATA. In: Egger M, Smith GD, Altman DG, editors. Systematic
Reviews in Health Care: Meta-analysis in context. London: BMJ Books;
2001. p. 347-69.
15. Santhanam I, Sangareddi S, Venkataraman S,
Kissoon N, Thiruvengadamudayan V. A prospective randomized controlled
study of two fluid regimens in the initial management of septic shock in
the emergency department. Pediatr Emerg Care. 2008;24:647-55.
16. Maitland K, George EC, Evans JA, Kiguli S,
Olupot-Olupot P, Akech SO, et al. Exploring mechanisms of excess
mortality with early fluid resuscitation: insights from the FEAST trial.
BMC Med. 2013;11:68.
17. Kiguli S1, Akech SO, Mtove G, Opoka RO, Engoru C,
Olupot-Olupot P, et al. WHO guidelines on fluid resuscitation in
children: missing the FEAST data. BMJ. 2014; 348:f7003.
18. American College of Chest Physicians/Society of
Critical Care Medicine Consensus Conference: Definitions for sepsis and
organ failure and guidelines for the use of innovative therapies in
sepsis. Crit Care Med. 1992;20:864-74.
19. Task force of the American College of Critical
Care Medicine, Society of Critical Care Medicine. Practice parameters
for hemodynamic support of sepsis in adults with sepsis. Crit Care Med.
1999;27:695-7.
20. Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, et al. Surviving Sepsis Campaign Guidelines
Committee including the Pediatric Subgroup. Surviving sepsis campaign:
international guidelines for management of severe sepsis and septic
shock: 2012. Crit Care Med. 2013;41:580-637.
21. de Oliveira CF, de Oliveira DS, Gottschald AF.
ACCM/PALS haemodynamic support guidelines for paediatric septic shock:
An outcomes comparison with and without monitoring central venous oxygen
saturation. Intensive Care Med. 2008; 34:1065-75.
22. Ford N, Hargreaves S, Shanks L. Mortality after
fluid bolus in children with shock due to sepsis or severe infection: A
systematic review and meta-analysis. PLoS One. 2012;7:e43953.
23. Opiyo N, Molyneux E, Sinclair D, Garner P,
English M. Immediate fluid management of children with severe febrile
illness and signs of impaired circulation in low-income settings: A
contextualised systematic review. BMJ Open. 2014; 4:e004934.
24. Duke T. What the African fluid-bolus trial means.
Lancet. 2011;378:1685-7.
25. Otiena H, Were E, Agmed I, Brent A, Maitland K.
Are bedside features of shock reproducible between different observers?
BMJ. 2004;89:979.
26. Scott H, Melendez E, Cruz AT; American Academy of
Pediatrics, Section on Emergency Medicine, Septic Shock Collaborative.
Mortality after fluid bolus in African children with sepsis. N Engl J
Med. 2011;365:1350-1.
27. Berend K. Mortality after fluid bolus in African
children with sepsis. N Engl J Med. 2011;365:1349.
28. Ford SR, Visram A. Mortality after fluid bolus in
African children with sepsis. N Engl J Med. 2011;365:1348.
29. Kissoon N, Carcillo JA, Global Sepsis Initiative
of the World Federation of Pediatric Intensive and Critical Care
Societies. Mortality after fluid bolus in African children with sepsis.
N Engl J Med. 2011;365:1350.
30. Joyner BL Jr, Boyd JM, Kocis KC. Mortality after
fluid bolus in African children with sepsis. N Engl J Med. 2011;
365:1349-50.
31. Duke T, Molyneux EM. Intravenous fluids for
seriously ill children: time to reconsider. Lancet. 2003;362:1320-3.
32. Dhawan A, Narang A, Singhi S. Hyponatremia and
the inappropriate ADH syndrome in pneumonia. Ann Trop Paediatr.
1992;12:455-62.
33. Duke T, Mokela D, Frank D. Management of
meningitis in children with oral fluid restriction or intravenous fluid
at maintenance volumes: a randomised trial. Ann Trop Pediatr.
2002;22:145-57.
|
|
|
 |
|

